NDC Code(s) : 64980-449-03, 64980-449-28, 64980-449-07, 64980-449-97
Packager : Rising Pharmaceuticals, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| fingolimodFingolimod hcl CAPSULE | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Rising Pharmaceuticals, Inc.(041241766) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Sunshine Lake Pharma Co., Ltd. | 545391443 | manufacture(64980-449) | |
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
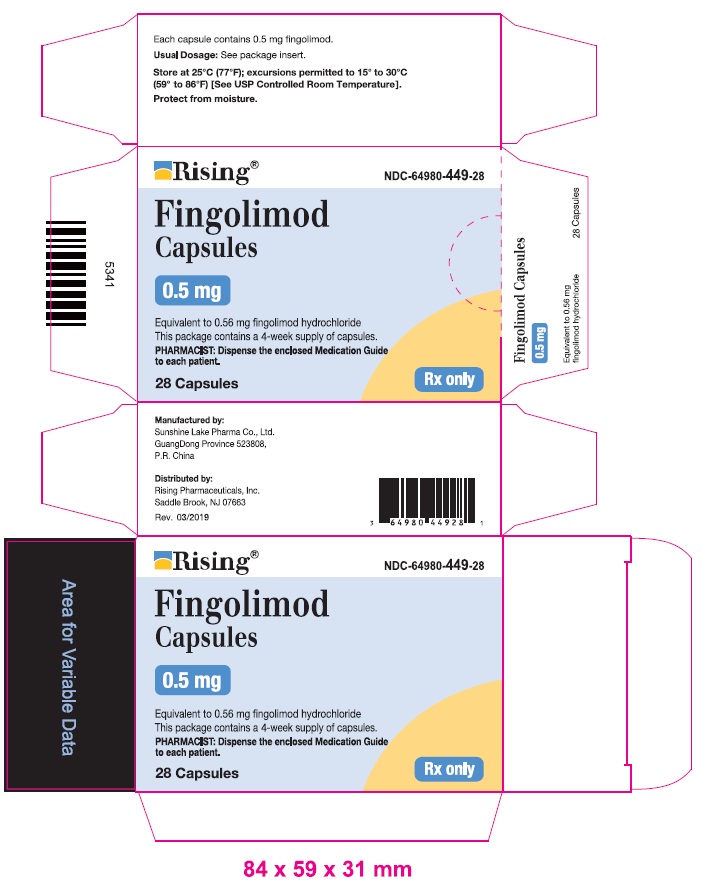
Package Label – 0.5 mg
Rx Only NDC64980-449-28
Fingolimod
Capsules
28 Capsules
0.5 mg
Equivalent to 0.56 mg fingolimod hydrochloride
This package contains a four-week supply of capsules.
Dispense with enclosed Medication Guide.
PRINCIPAL DISPLAY PANEL
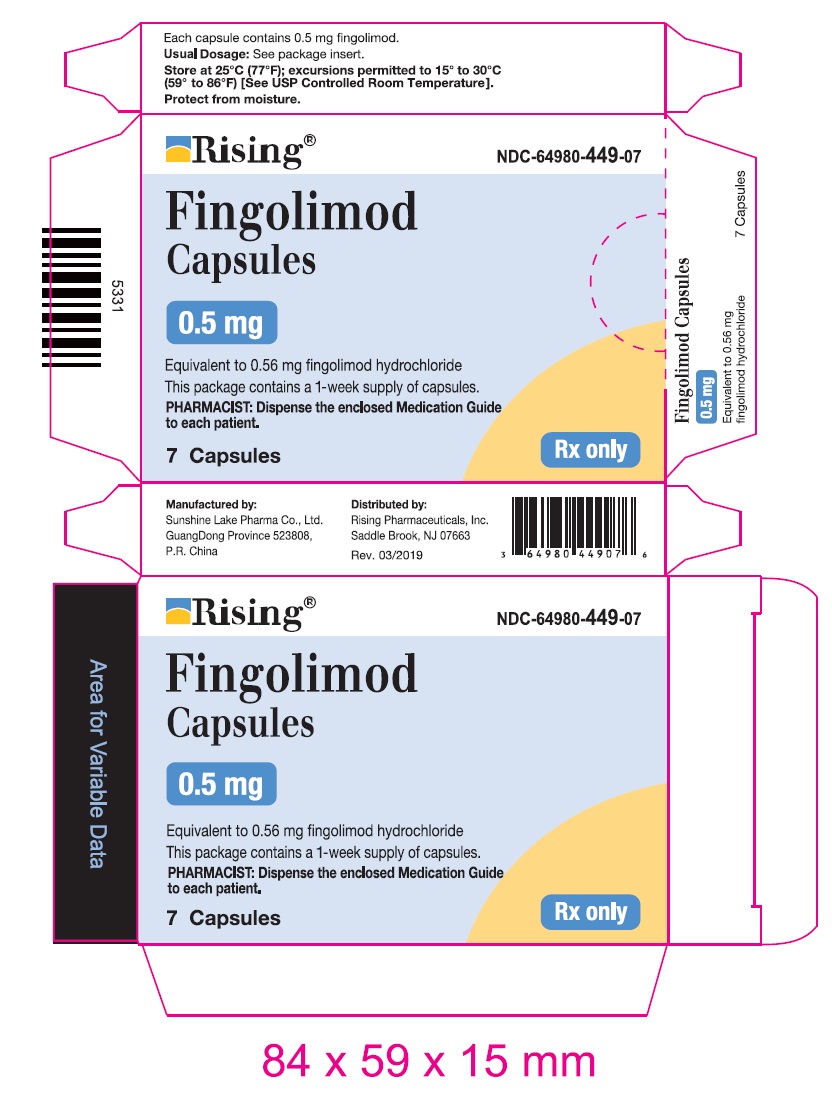
Package Label – 0.5 mg
Rx Only NDC 64980-449-07
Fingolimod
Capsules
7 Capsules
0.5 mg
Equivalent to 0.56 mg fingolimod hydrochloride
Dispense with enclosed Medication Guide.
PRINCIPAL DISPLAY PANEL
Package Label – 0.5 mg
Rx Only NDC 64980-449-03
Fingolimod
Capsules
30 Capsules
0.5 mg
Equivalent to 0.56 mg fingolimod hydrochloride
Dispense with enclosed Medication Guide.










