NDC Code(s) : 62332-549-15, 62332-549-45, 62332-549-46
Packager : Alembic Pharmaceuticals Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| ADAPALENEADAPALENE GEL | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| LABELER - Alembic Pharmaceuticals Inc.(079288842) |
| REGISTRANT - Alembic Pharmaceuticals Limited(650574663) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Alembic Pharmaceuticals Limited | 871411532 | MANUFACTURE(62332-549), ANALYSIS(62332-549) | |
PRINCIPAL DISPLAY PANEL
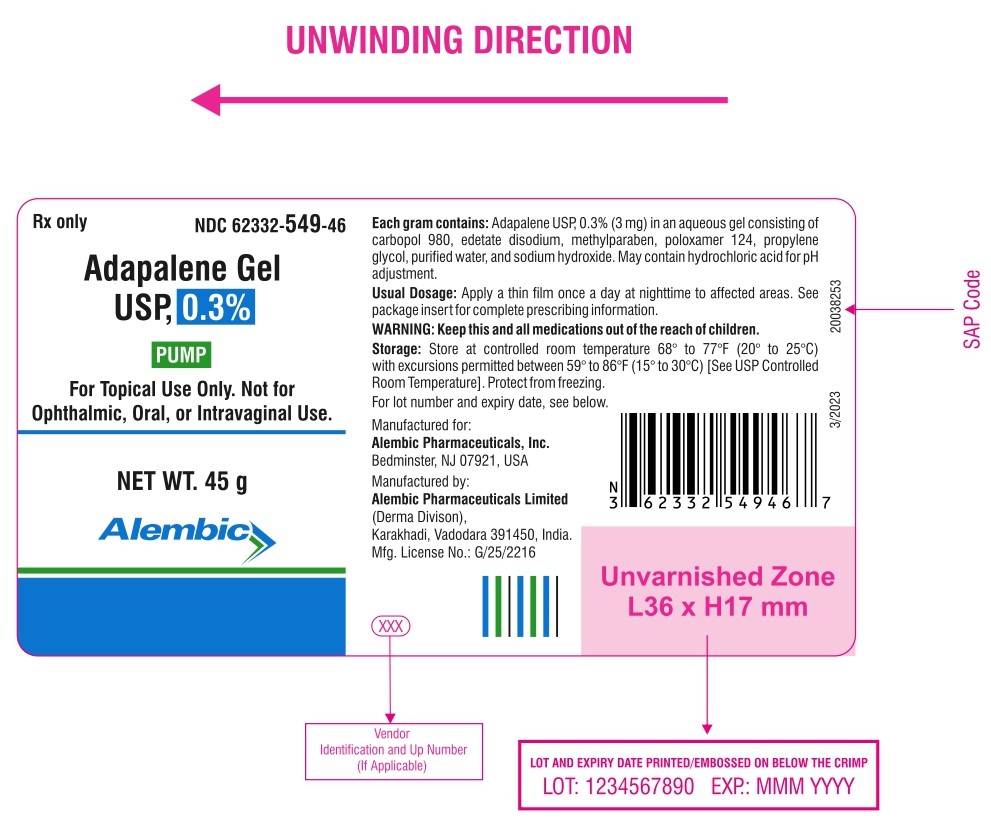
Rx only
NDC 62332-549-46
Adapalene Gel USP, 0.3%
PUMP
FOR TOPICAL USE ONLY
NET WT.45 g
Alembic
For topical use only. Not for ophthalmic, oral, or intravaginal use.
Usual dosage: apply a thin film once a day at nighttime to affected areas. See package insert for complete prescribing information.
Each gram contains: adapalene USP, 0.3% (3 mg) in an aqueous gel consisting of carbopol 980, edetate disodium, methylparaben, poloxamer 124, propylene glycol, purified water, and sodium hydroxide. May contain hydrochloric acid for pH adjustment.
Storage: Store at controlled room temperature 68° to 77°F (20° to 25°C) with excursions permitted between 59° to 86°F (15° to 30°C) [See USP Controlled Room Temperature]. Protect from freezing.
For lot number and expiration date see below the crimp of Pump.
Manufactured for:
Alembic Pharmaceuticals, Inc.
Bedminster, NJ 07921, USA
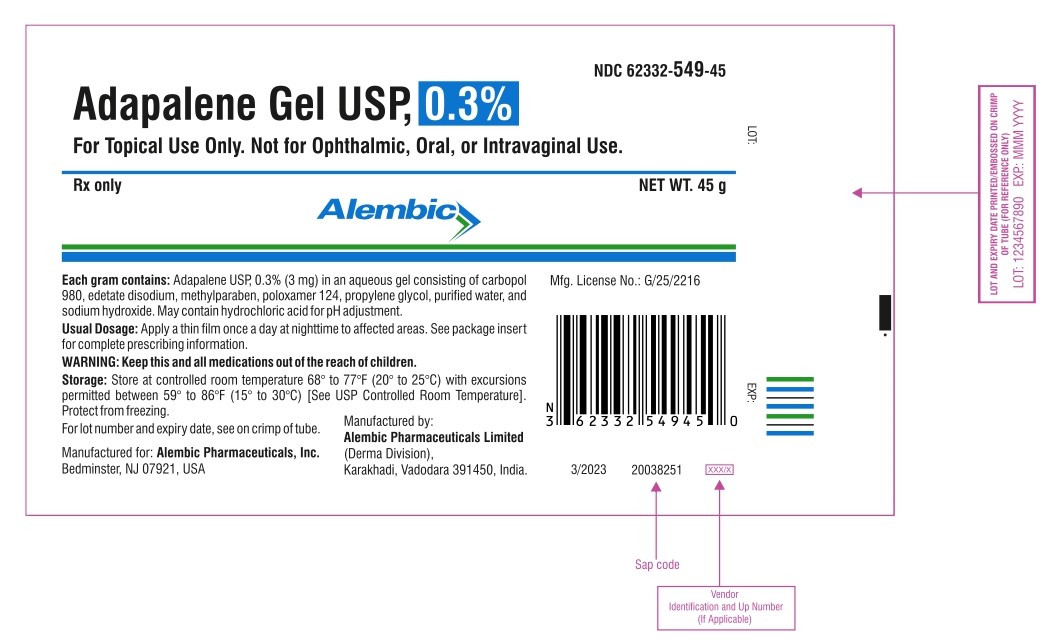

Rx only
NDC 62332-549-45
Adapalene Gel USP, 0.3%
TUBE
FOR TOPICAL USE ONLY
NET WT.45 g
Alembic
For topical use only. Not for ophthalmic, oral, or intravaginal use.
Usual dosage: apply a thin film once a day at nighttime to affected areas. See package insert for complete prescribing information.
Each gram contains: adapalene USP, 0.3% (3 mg) in an aqueous gel consisting of carbopol 980, edetate disodium, methylparaben, poloxamer 124, propylene glycol, purified water, and sodium hydroxide. May contain hydrochloric acid for pH adjustment.
Storage: Store at controlled room temperature 68° to 77°F (20° to 25°C) with excursions permitted between 59° to 86°F (15° to 30°C) [See USP Controlled Room Temperature]. Protect from freezing.
For lot number and expiration date, see on crimp of tube.
Manufactured for:
Alembic Pharmaceuticals, Inc.
Bedminster, NJ 07921, USA









