NDC Code(s) : 62037-839-20, 62037-849-20, 62037-861-20, 62037-862-20, 62037-863-20, 62037-864-20, 62037-866-20
Packager : Actavis Pharma, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Enoxaparin SodiumEnoxaparin Sodium INJECTION | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Enoxaparin SodiumEnoxaparin Sodium INJECTION | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Enoxaparin SodiumEnoxaparin Sodium INJECTION | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Enoxaparin SodiumEnoxaparin Sodium INJECTION | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Enoxaparin SodiumEnoxaparin Sodium INJECTION | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Enoxaparin SodiumEnoxaparin Sodium INJECTION | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Enoxaparin SodiumEnoxaparin Sodium INJECTION | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
NDC 62037-839-20
ENOXAPARIN SODIUM INJECTION
30 mg/0.3 mL
Rx ONLY
FOR SUBCUTANEOUS INJECTION
10 x 30 mg Single Dose Syringes with
Automatic Safety Device
PRINCIPAL DISPLAY PANEL
NDC 62037-849-20
ENOXAPARIN SODIUM INJECTION
40 mg/0.4 mL
Rx ONLY
FOR SUBCUTANEOUS INJECTION
10 x 40 mg Single Dose Syringes with
Automatic Safety Device
PRINCIPAL DISPLAY PANEL
NDC 62037-861-20
ENOXAPARIN SODIUM INJECTION
60 mg/0.6 mL
Rx ONLY
FOR SUBCUTANEOUS INJECTION
10 x 60 mg Single Dose Syringes with
Automatic Safety Device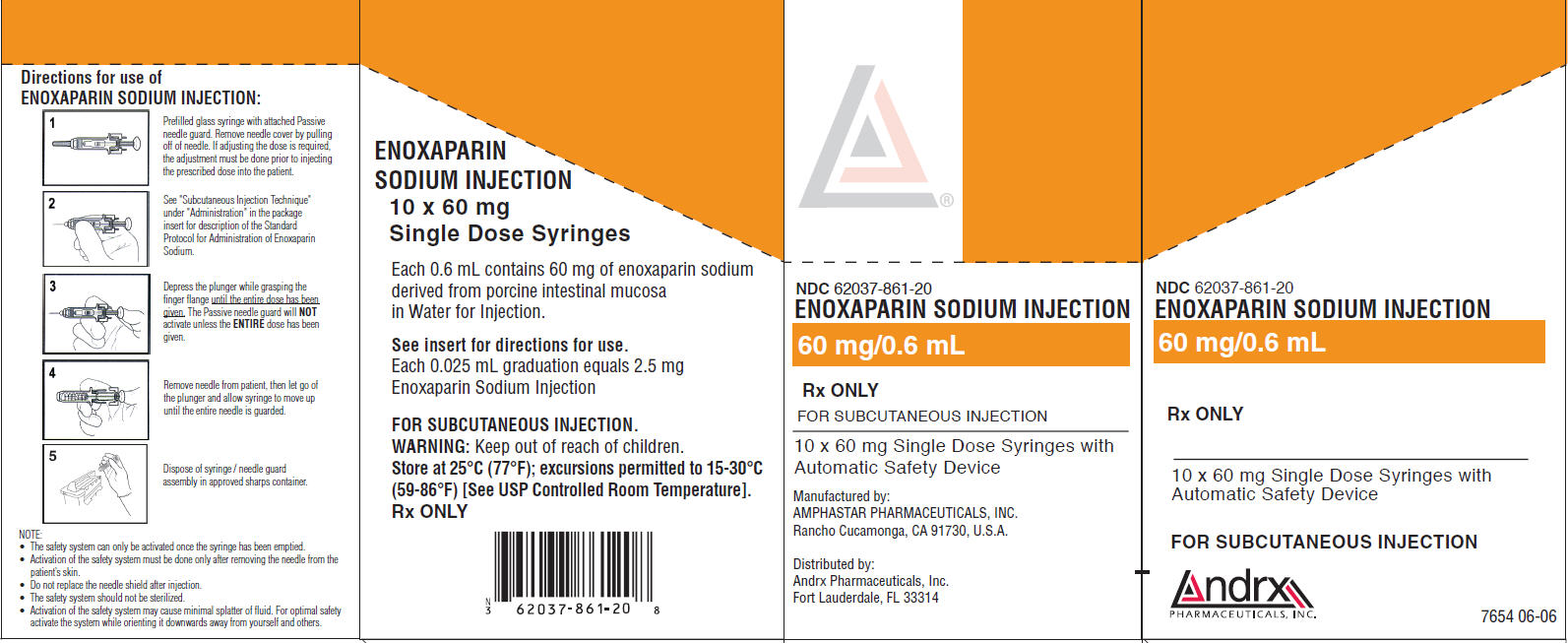
PRINCIPAL DISPLAY PANEL
NDC 62037-862-20
ENOXAPARIN SODIUM INJECTION
80 mg/0.8 mL
Rx ONLY
FOR SUBCUTANEOUS INJECTION
10 x 80 mg Single Dose Syringes with
Automatic Safety Device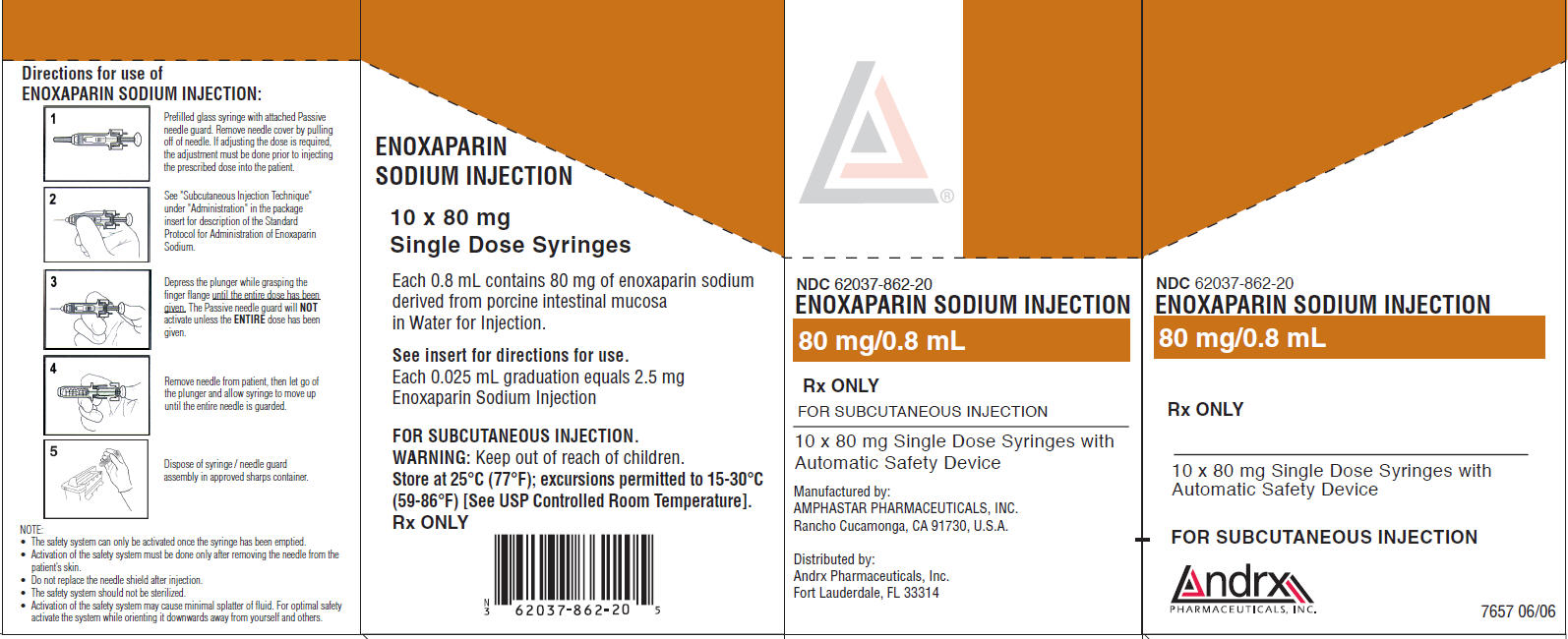
PRINCIPAL DISPLAY PANEL
NDC 62037-863-20
ENOXAPARIN SODIUM INJECTION
100 mg/ mL
Rx ONLY
1 mL Syringe
FOR SUBCUTANEOUS INJECTION
10 x 100 mg Single Dose Syringes with
Automatic Safety Device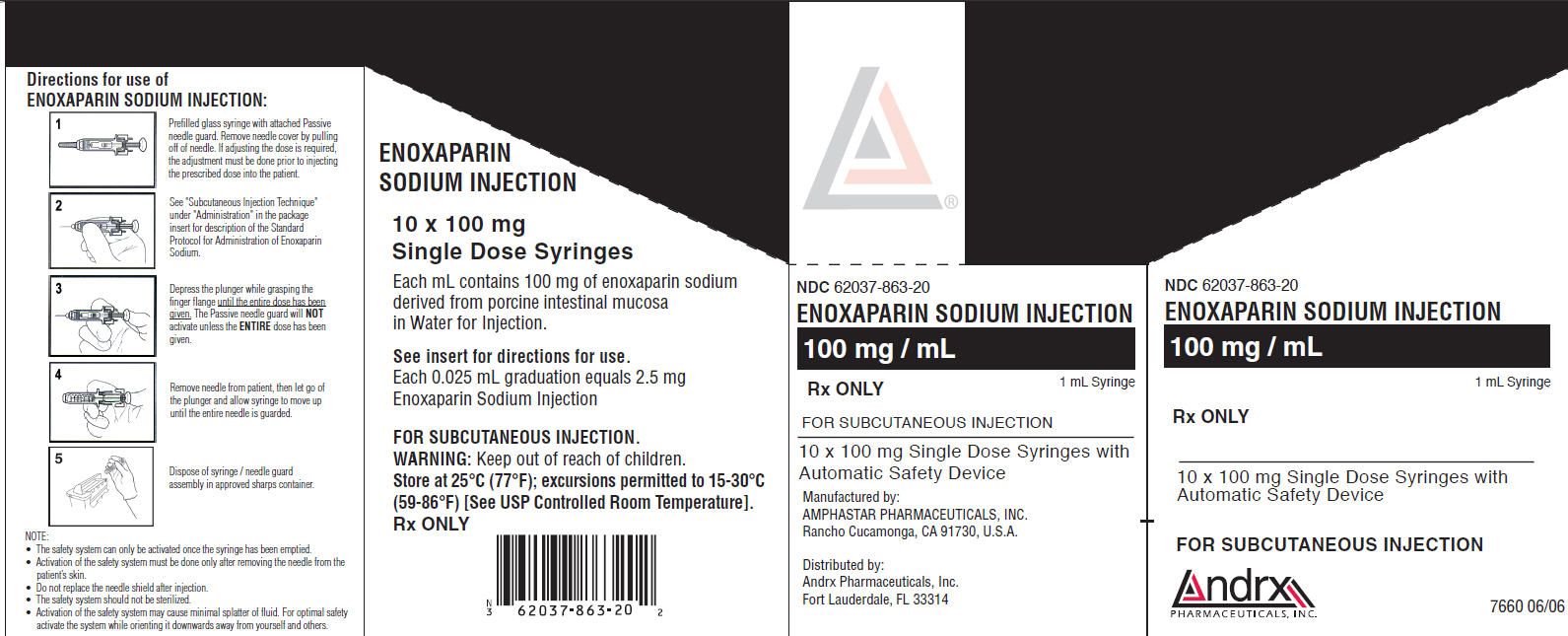
PRINCIPAL DISPLAY PANEL
NDC 62037-864-20
ENOXAPARIN SODIUM INJECTION
120 mg/0.8 mL
Rx ONLY
FOR SUBCUTANEOUS INJECTION
10 x 120 mg Single Dose Syringes with
Automatic Safety Device
PRINCIPAL DISPLAY PANEL
NDC 62037-866-20
ENOXAPARIN SODIUM INJECTION
150 mg/ mL
Rx ONLY
1 mL Syringe
FOR SUBCUTANEOUS INJECTION
10 x 150 mg Single Dose Syringes with
Automatic Safety Device








