NDC Code(s) : 60505-6142-0, 60505-6142-5, 60505-6231-0, 60505-6231-5, 60505-6266-0, 60505-6266-5
Packager : Apotex Corp.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CefazolinCefazolin INJECTION, POWDER, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CefazolinCefazolin INJECTION, POWDER, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CefazolinCefazolin INJECTION, POWDER, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Apotex Corp.(845263701) |
| REGISTRANT - Qilu Pharmaceutical Co., Ltd.(653878256) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Qilu Pharmaceutical Co., Ltd.(High Tech Zone Site) | 421279342 | manufacture(60505-6142, 60505-6231, 60505-6266), analysis(60505-6142, 60505-6231, 60505-6266) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Qilu Antibiotics Pharmaceutical Co., Ltd. | 527271779 | manufacture(60505-6142, 60505-6231), analysis(60505-6142, 60505-6231) | |
PRINCIPAL DISPLAY PANEL
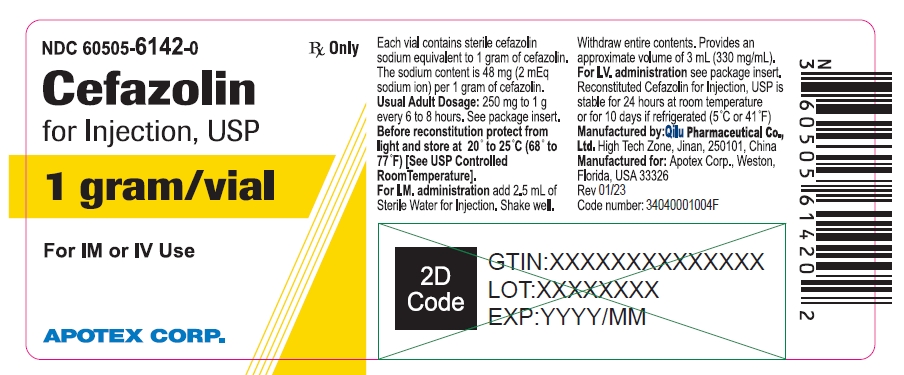
NDC 60505-6142-0
Cefazolin for Injection, USP
1 gram/vial
For IM or IV Use
Rx Only
APOTEX CORP.

PRINCIPAL DISPLAY PANEL
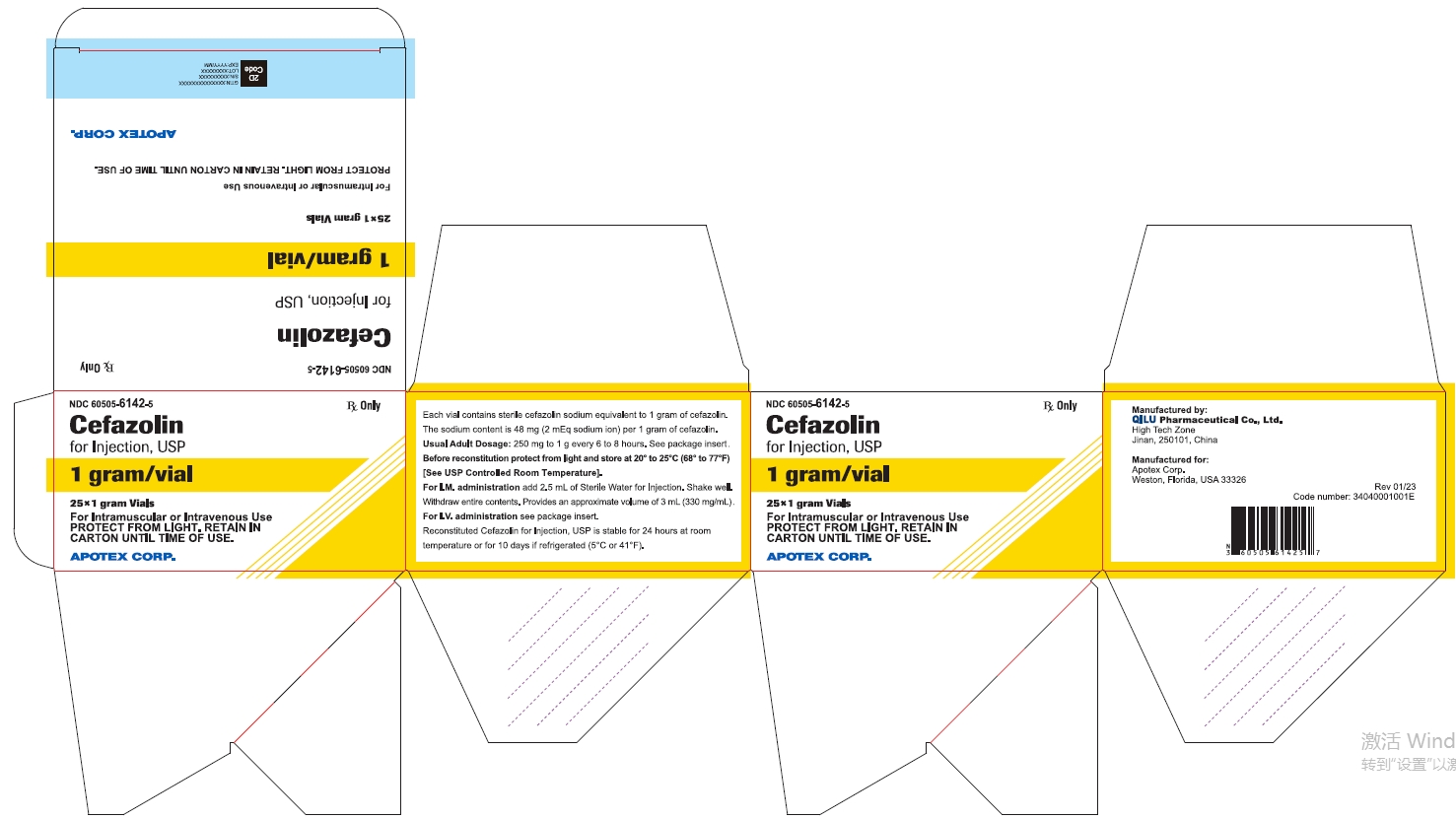
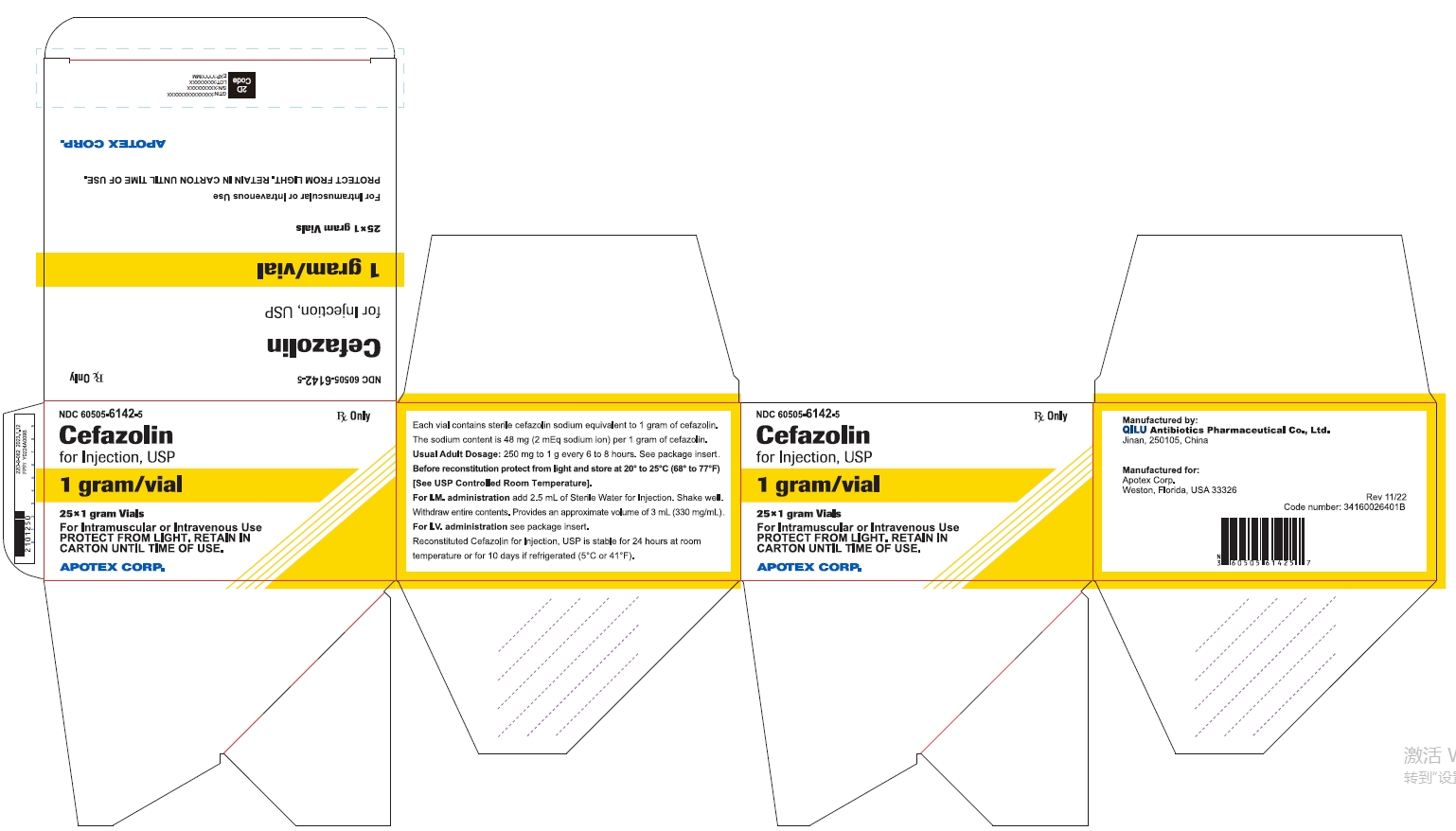
25* 1 gram Vials NDC 60505-6142-5
Cefazolin for Injection, USP
1 gram/vial
For Intramuscular or Intravenous Use
PROTECT FROM LIGHT. RETAIN IN CARTON UNTIL TIME OF USE
APOTEX CORP.
PRINCIPAL DISPLAY PANEL
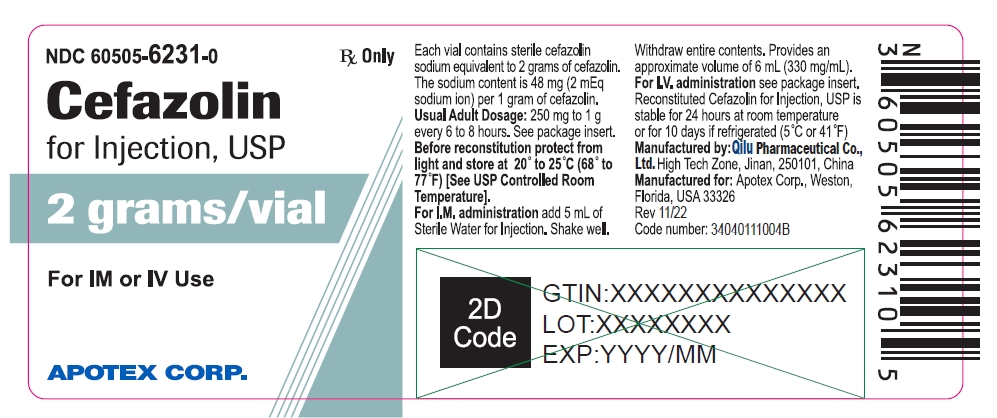
NDC 60505-6231-0
Cefazolin for Injection, USP
2 grams/vial
For IM or IV Use
Rx Only
APOTEX CORP.

PRINCIPAL DISPLAY PANEL
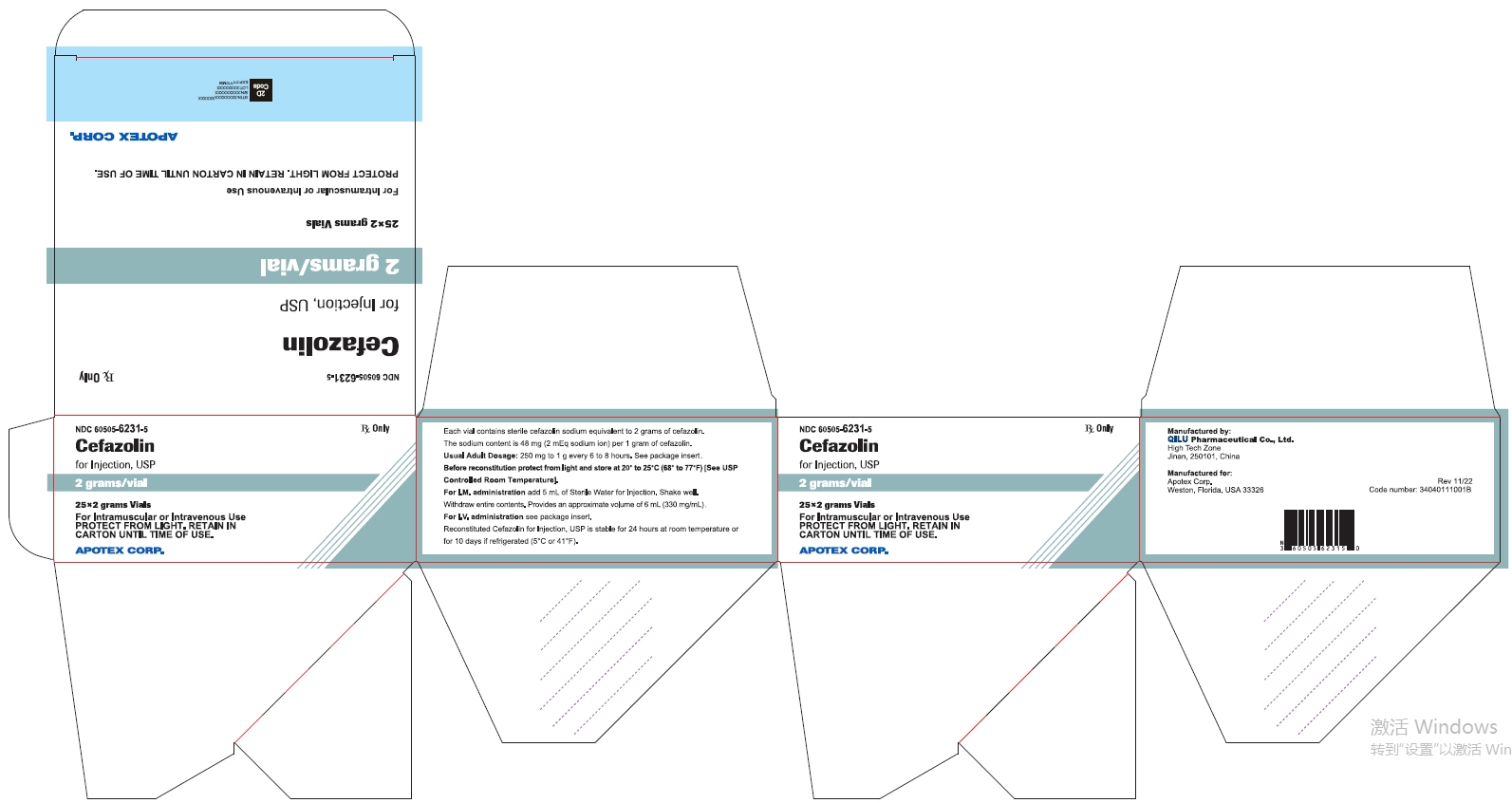
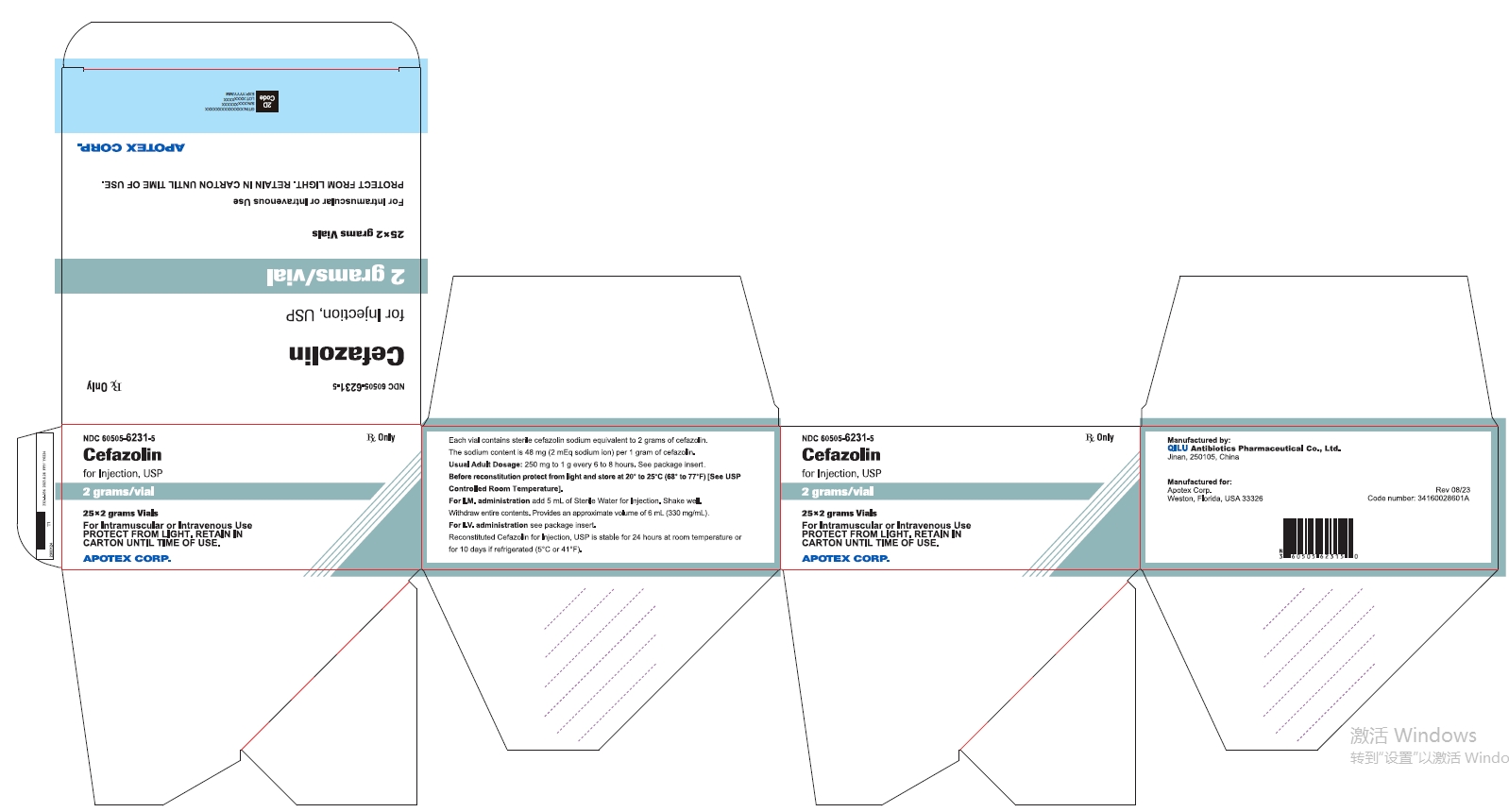
25* 2 grams Vials NDC 60505-6231-5
Cefazolin for Injection, USP
2 grams/vial
For Intramuscular or Intravenous Use
PROTECT FROM LIGHT. RETAIN IN CARTON UNTIL TIME OF USE
Rx Only
APOTEX CORP.
PRINCIPAL DISPLAY PANEL
NDC 60505-6266-0
Cefazolin for Injection, USP
3 grams/vial
For IM or IV Use
Rx Only
APOTEX CORP.

PRINCIPAL DISPLAY PANEL
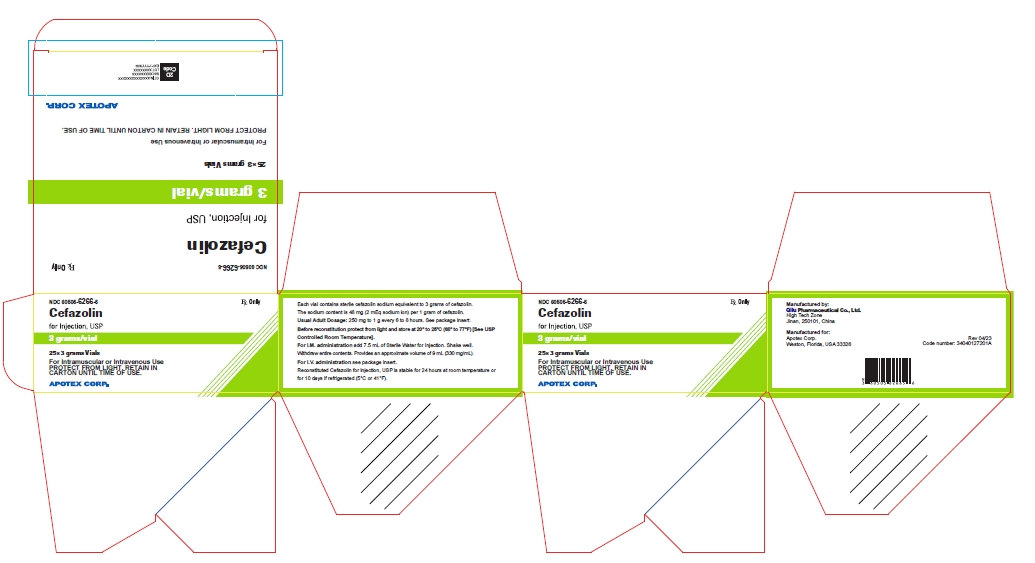
25* 3 grams Vials NDC 60505-6266-5
Cefazolin for Injection, USP
3 grams/vial
For Intramuscular or Intravenous Use
PROTECT FROM LIGHT. RETAIN IN CARTON UNTIL TIME OF USE
Rx Only
APOTEX CORP.