NDC Code(s) : 59385-021-01, 59385-021-60, 59385-022-01, 59385-022-60, 59385-023-01, 59385-023-60, 59385-024-01, 59385-024-60, 59385-025-01, 59385-025-60, 59385-026-01, 59385-026-60, 59385-027-01, 59385-027-60
Packager : BioDelivery Sciences International Inc
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CIII
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| BELBUCAbuprenorphine hydrochloride FILM, SOLUBLE | ||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| BELBUCAbuprenorphine hydrochloride FILM, SOLUBLE | ||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| BELBUCAbuprenorphine hydrochloride FILM, SOLUBLE | ||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| BELBUCAbuprenorphine hydrochloride FILM, SOLUBLE | ||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| BELBUCAbuprenorphine hydrochloride FILM, SOLUBLE | ||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| BELBUCAbuprenorphine hydrochloride FILM, SOLUBLE | ||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| BELBUCAbuprenorphine hydrochloride FILM, SOLUBLE | ||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| LABELER - BioDelivery Sciences International Inc(016058955) |
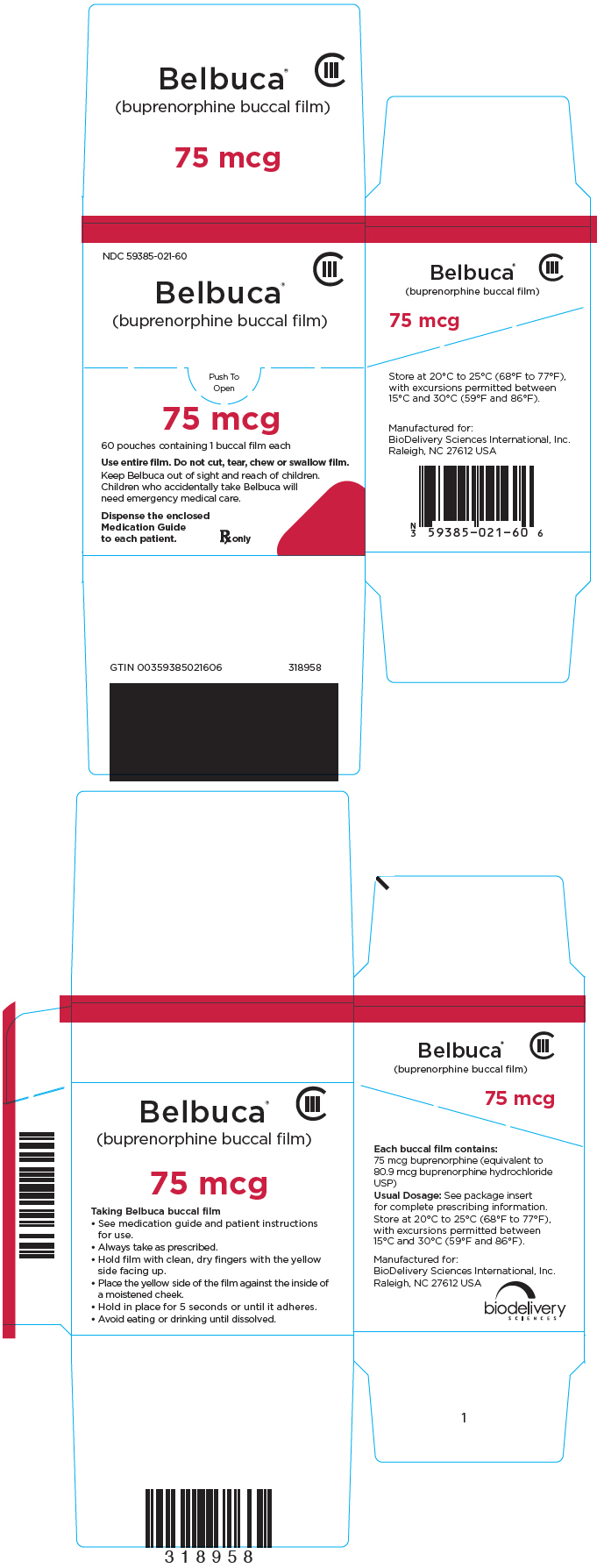
PRINCIPAL DISPLAY PANEL
NDC 59385-021-60
CIII
Belbuca
®
(buprenorphine buccal film)
Push To
Open
75 mcg
60 pouches containing 1 buccal film each
Use entire film. Do not cut, tear, chew or swallow film.
Keep Belbuca out of sight and reach of children.
Children who accidentally take Belbuca will
need emergency medical care.
Dispense the enclosed
Medication Guide
to each patient.
Rx only
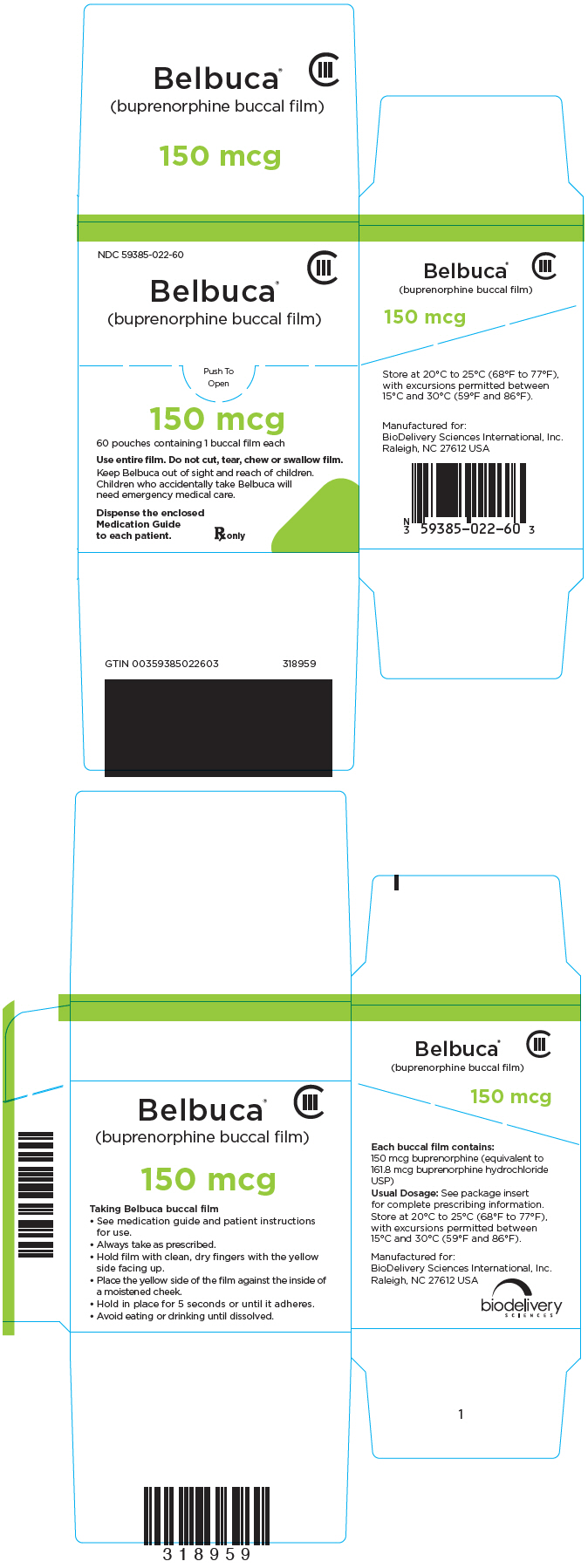
PRINCIPAL DISPLAY PANEL
NDC 59385-022-60
CIII
Belbuca
®
(buprenorphine buccal film)
Push To
Open
150 mcg
60 pouches containing 1 buccal film each
Use entire film. Do not cut, tear, chew or swallow film.
Keep Belbuca out of sight and reach of children.
Children who accidentally take Belbuca will
need emergency medical care.
Dispense the enclosed
Medication Guide
to each patient.
Rx only
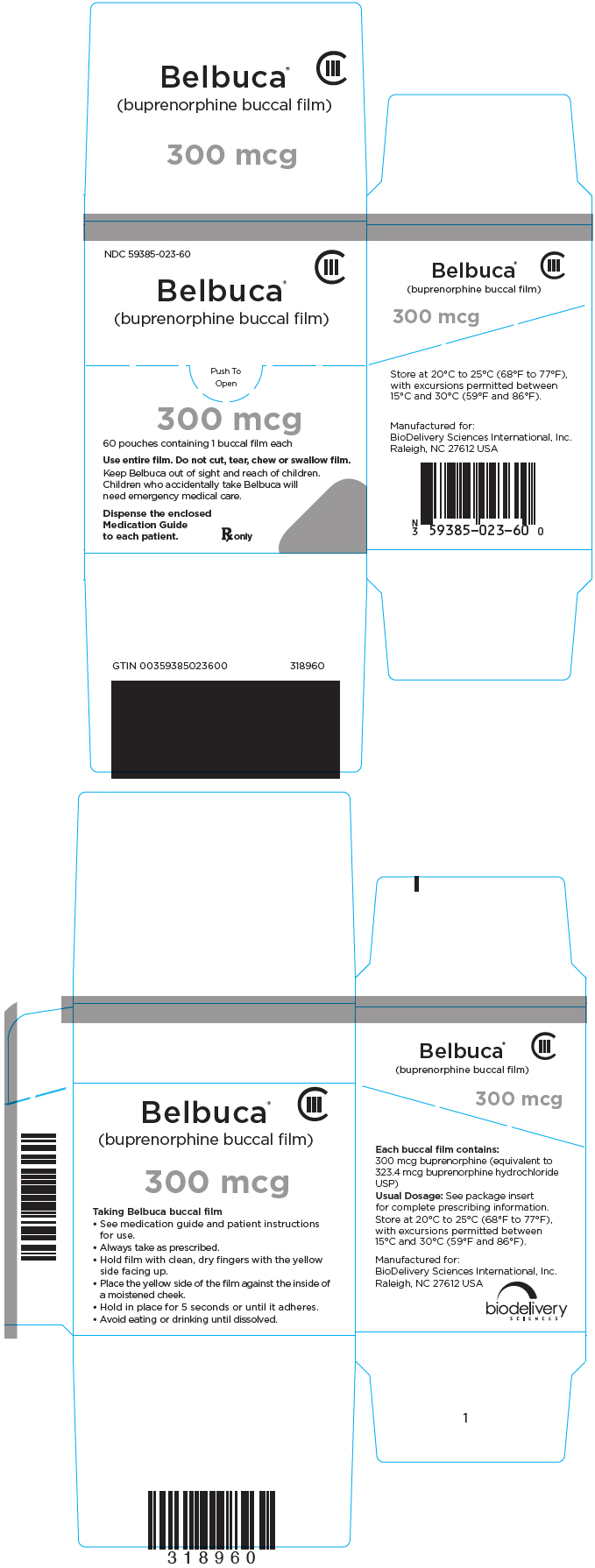
PRINCIPAL DISPLAY PANEL
NDC 59385-023-60
CIII
Belbuca
®
(buprenorphine buccal film)
Push To
Open
300 mcg
60 pouches containing 1 buccal film each
Use entire film. Do not cut, tear, chew or swallow film.
Keep Belbuca out of sight and reach of children.
Children who accidentally take Belbuca will
need emergency medical care.
Dispense the enclosed
Medication Guide
to each patient.
Rx only
PRINCIPAL DISPLAY PANEL
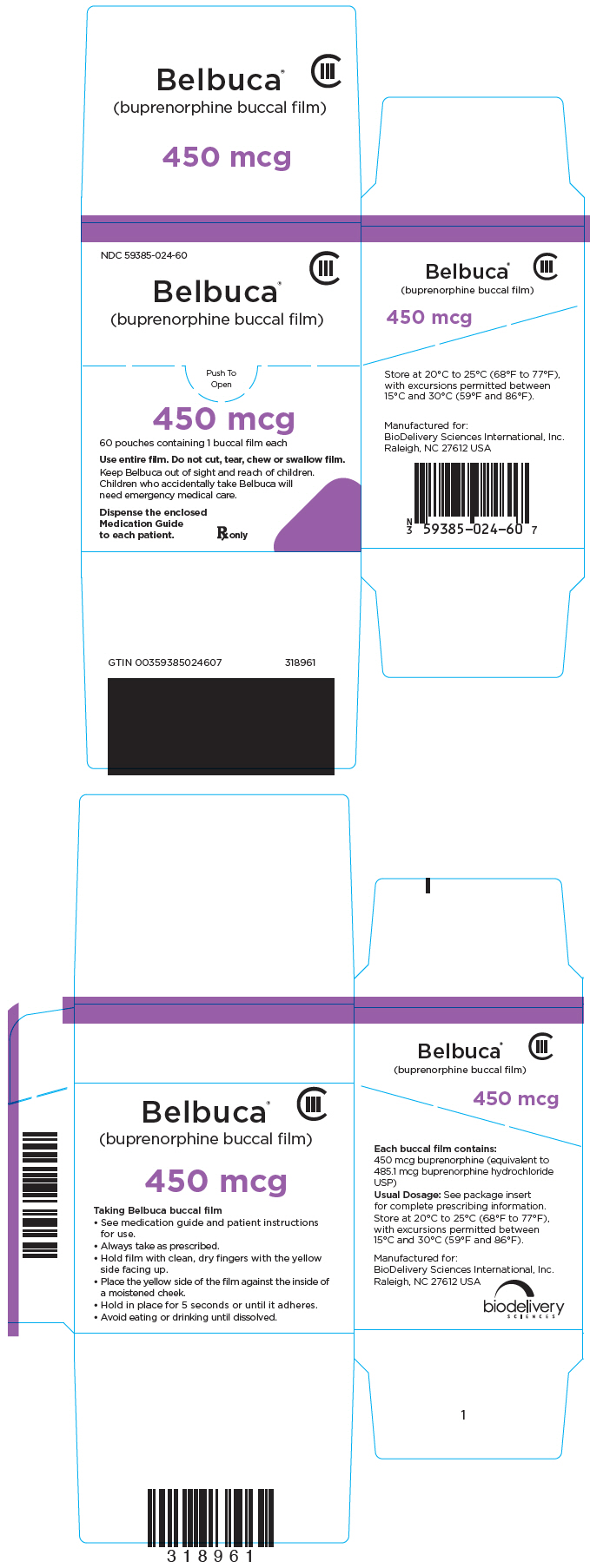
NDC 59385-024-60
CIII
Belbuca
®
(buprenorphine buccal film)
Push To
Open
450 mcg
60 pouches containing 1 buccal film each
Use entire film. Do not cut, tear, chew or swallow film.
Keep Belbuca out of sight and reach of children.
Children who accidentally take Belbuca will
need emergency medical care.
Dispense the enclosed
Medication Guide
to each patient.
Rx only
PRINCIPAL DISPLAY PANEL
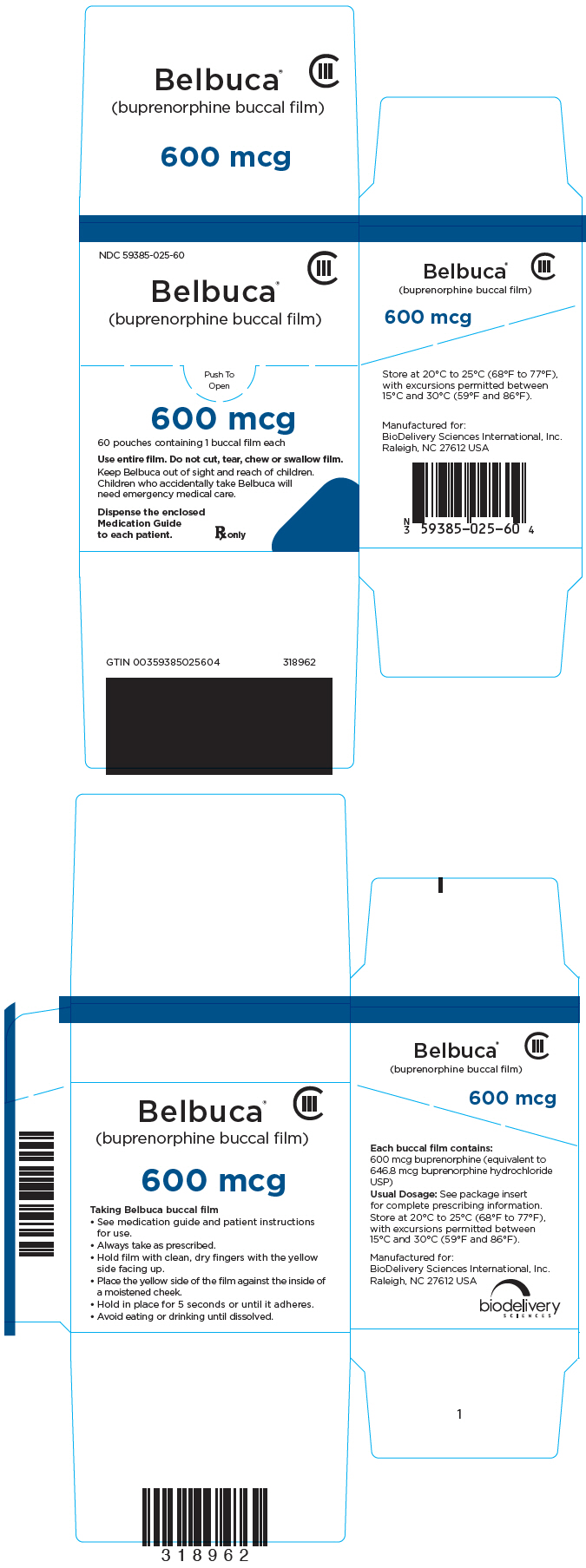
NDC 59385-025-60
CIII
Belbuca
®
(buprenorphine buccal film)
Push To
Open
600 mcg
60 pouches containing 1 buccal film each
Use entire film. Do not cut, tear, chew or swallow film.
Keep Belbuca out of sight and reach of children.
Children who accidentally take Belbuca will
need emergency medical care.
Dispense the enclosed
Medication Guide
to each patient.
Rx only
PRINCIPAL DISPLAY PANEL
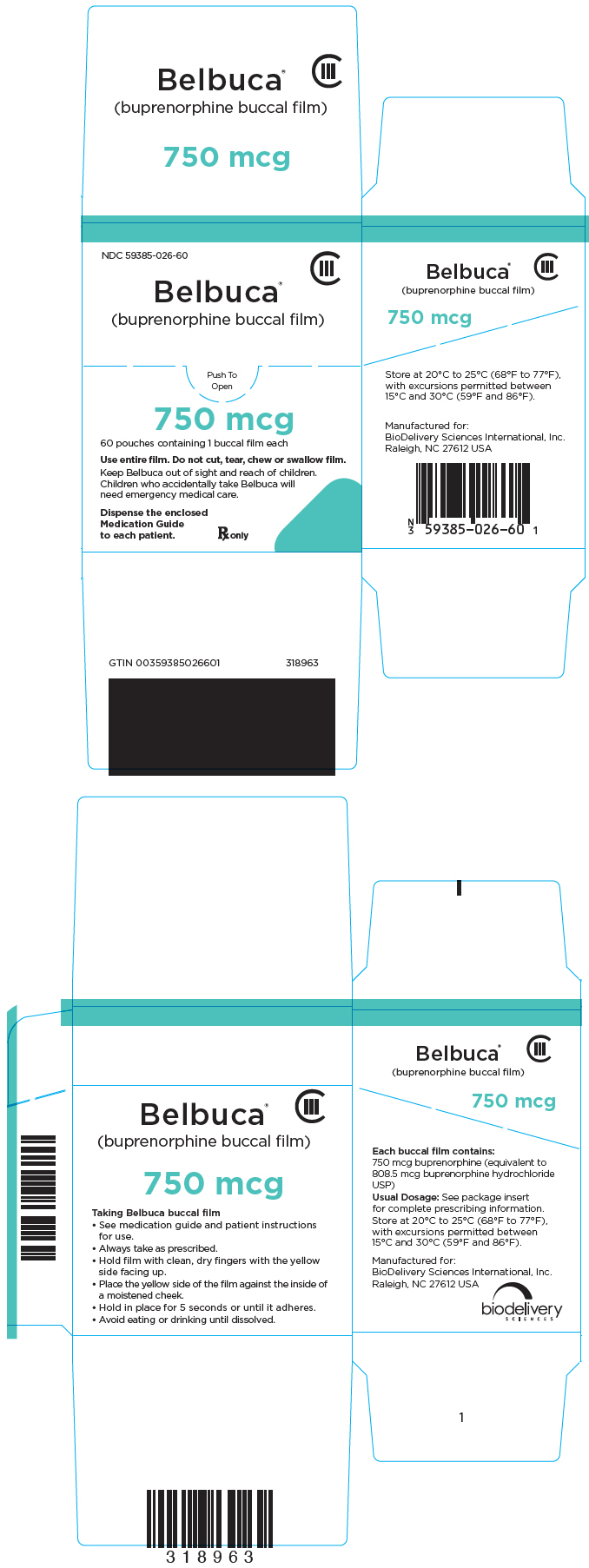
NDC 59385-026-60
CIII
Belbuca
®
(buprenorphine buccal film)
Push To
Open
750 mcg
60 pouches containing 1 buccal film each
Use entire film. Do not cut, tear, chew or swallow film.
Keep Belbuca out of sight and reach of children.
Children who accidentally take Belbuca will
need emergency medical care.
Dispense the enclosed
Medication Guide
to each patient.
Rx only
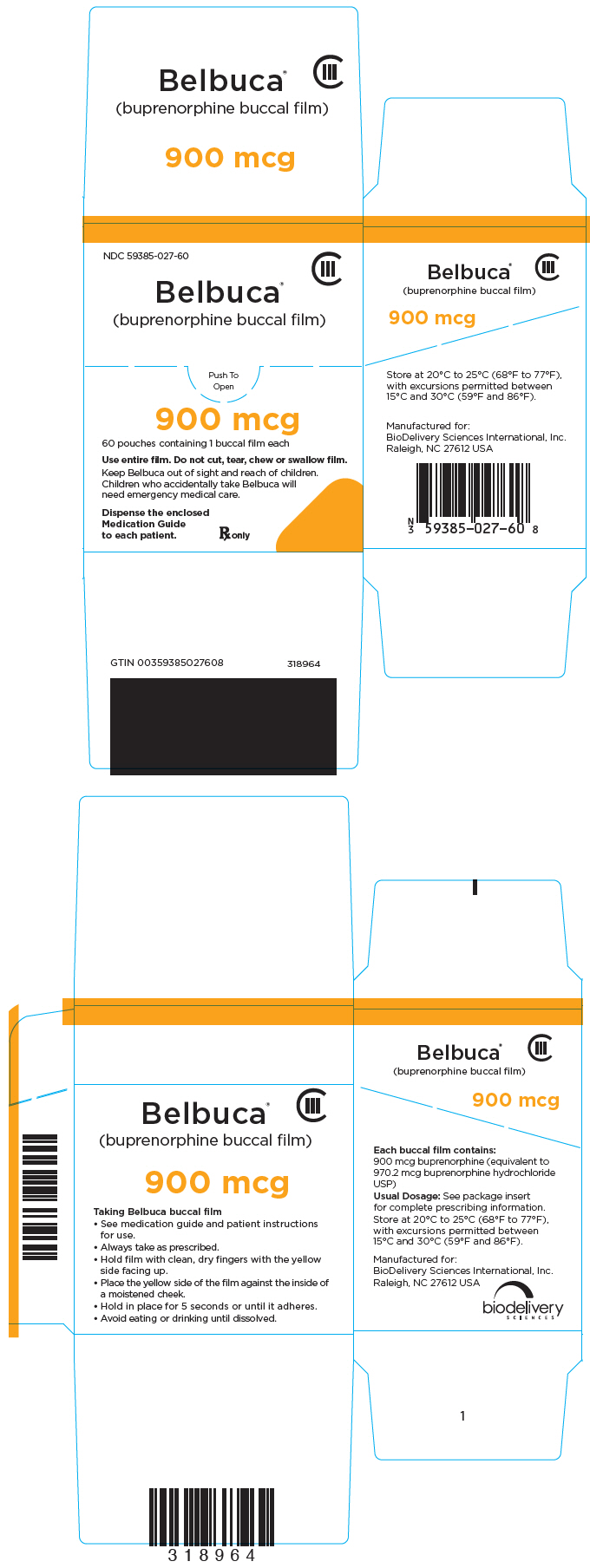
PRINCIPAL DISPLAY PANEL
NDC 59385-027-60
CIII
Belbuca
®
(buprenorphine buccal film)
Push To
Open
900 mcg
60 pouches containing 1 buccal film each
Use entire film. Do not cut, tear, chew or swallow film.
Keep Belbuca out of sight and reach of children.
Children who accidentally take Belbuca will
need emergency medical care.
Dispense the enclosed
Medication Guide
to each patient.
Rx only