NDC Code(s) : 57894-502-05, 57894-502-20, 57894-505-05, 57894-505-20
Packager : Janssen Biotech, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| DARZALEXDaratumumab INJECTION, SOLUTION, CONCENTRATE | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Darzalex IVDaratumumab INJECTION, SOLUTION, CONCENTRATE | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Janssen Biotech, Inc.(099091753) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Janssen Biotech, Inc. | 038978363 | analysis(57894-502, 57894-505) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| AndersonBrecon, Inc. | 053217022 | pack(57894-502) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| FUJIFILM Diosynth Biotechnologies Denmark ApS | 307258082 | api manufacture(57894-505) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Vetter Pharma Fertigung GmbH & Co. KG | 312670654 | manufacture(57894-502), analysis(57894-502) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Vetter Pharma Fertigung GmbH & Co. KG | 316126754 | analysis(57894-502) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Vetter Pharma Fertigung GmbH & Co. KG | 341629292 | analysis(57894-502) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Vetter Pharma Fertigung GmbH & Co. KG | 344217323 | analysis(57894-502) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Janssen Biologics B.V. | 409612918 | analysis(57894-502, 57894-505) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Cilag AG | 483237103 | manufacture(57894-502, 57894-505), pack(57894-502, 57894-505), label(57894-502, 57894-505), analysis(57894-502, 57894-505) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Samsung Biologics Co, LTD | 557810567 | api manufacture(57894-502), analysis(57894-502) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Baxter Pharmaceutical Solutions, LLC | 604719430 | manufacture(57894-502), label(57894-502), pack(57894-502) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Biogen, MA Inc. | 841087823 | api manufacture(57894-502) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| PPD Development Ireland Ltd. | 985036175 | analysis(57894-502) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Janssen Sciences Ireland Unlimited Company | 986030167 | analysis(57894-502, 57894-505) | |
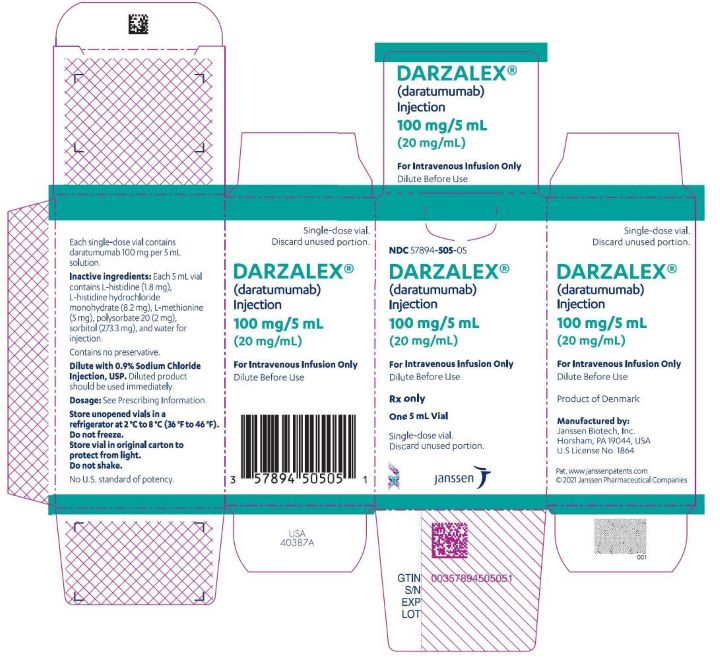
PRINCIPAL DISPLAY PANEL
NDC 57894-502-05
DARZALEX
®
(daratumumab)
Injection
100 mg/5 mL
(20 mg/mL)
For Intravenous Infusion Only
Dilute Before Use
Rx only
Single-dose vial.
Discard Unused Portion
janssen

PRINCIPAL DISPLAY PANEL
NDC 57894-505-05
DARZALEX
®
(daratumumab)
Injection
100 mg/5 mL
(20 mg/mL)
For Intravenous Infusion Only
Dilute Before Use
Rx only
One 5 mL Vial
Single-dose vial.
Discard unused portion.
janssen