NDC Code(s) : 55154-4706-1, 55154-4707-1, 55154-4708-1
Packager : Cardinal Health
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CII
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| MS CONTINmorphine sulfate TABLET, FILM COATED, EXTENDED RELEASE | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| MS CONTINmorphine sulfate TABLET, FILM COATED, EXTENDED RELEASE | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| MS CONTINmorphine sulfate TABLET, FILM COATED, EXTENDED RELEASE | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
NDC 59011-261-25 (Purdue Pharma L.P.)
MS Contin
(morphine sulfate controlled-release tablets)
30 mg
100 Tablets
Each controlled-release tablet contains: 30 mg morphine sulfate
Purdue Pharma L.P.
Rx Only
Warning: Keep out of reach of children.
CII

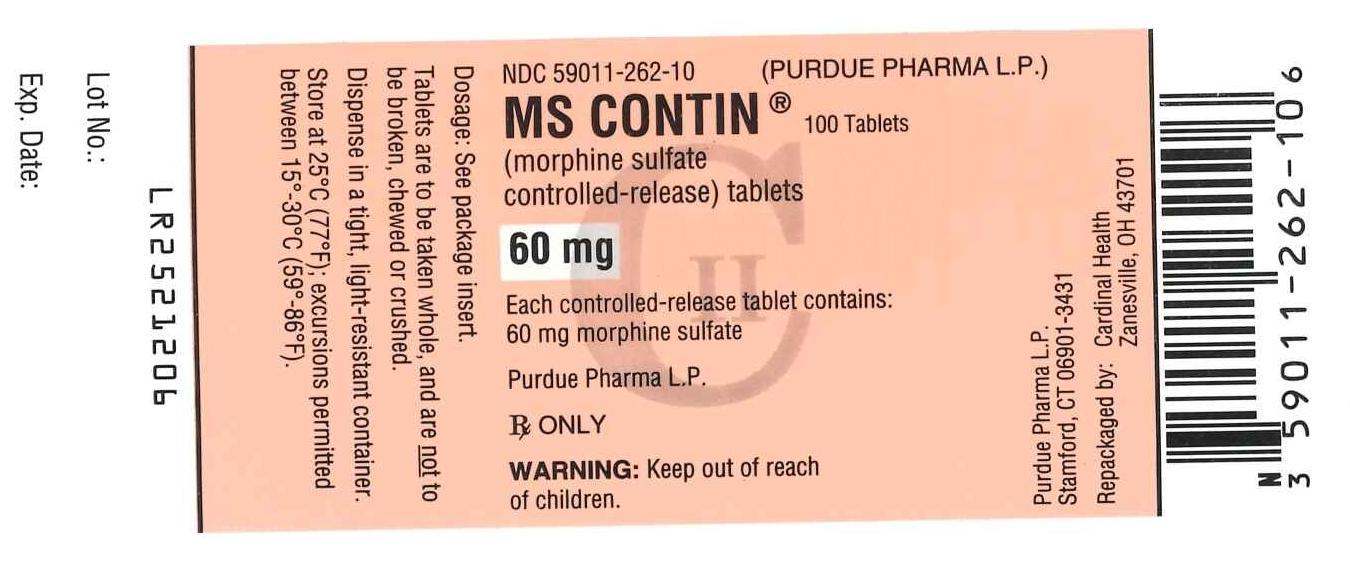
PRINCIPAL DISPLAY PANEL
NDC 59011-262-10 (Purdue Pharma L.P.)
MS Contin
(morphine sulfate controlled-release) tablets
60 mg
100 Tablets
Each controlled-release tablet contains: 60 mg morphine sulfate
Purdue Pharma L.P.
Rx Only
Warning: Keep out of reach of children.
CII
PRINCIPAL DISPLAY PANEL
NDC 59011-263-10 (Purdue Pharma L.P.)
MS Contin
100 Tablets
(morphine sulfate controlled-release tablets)
100 mg
100 Tablets
Each controlled-release tablet contains: 100 mg morphine sulfate
Purdue Pharma L.P.
Rx Only
Warning: Keep out of reach of children.
CII









