NDC Code(s) : 52817-380-10, 52817-381-10
Packager : TruPharma LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| NaproxenNaproxen TABLET, DELAYED RELEASE | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| NaproxenNaproxen TABLET, DELAYED RELEASE | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| LABELER - TruPharma LLC(078533947) |
| REGISTRANT - Tulex Pharmaceuticals Inc.(080119240) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Tulex Pharmaceuticals Inc. | 080119240 | analysis(52817-380, 52817-381), label(52817-380, 52817-381), manufacture(52817-380, 52817-381), pack(52817-380, 52817-381) | |
PRINCIPAL DISPLAY PANEL
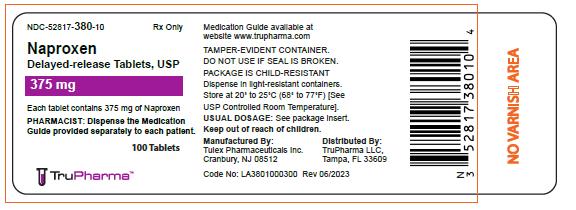
NDC 52817-380-10
Rx Only
Naproxen
Delayed-Release Tablets, USP
375 mg
PHARMACIST: Dispense the Medication Guide provided separately to each patient
100 Tablets
Medication Guide availbale at website www.trupharma.com
TAMPER-EVIDENT CONTAINER.
DO NOT USE IF SEAL IS BROKEN
PACKAGE IS CHILD-RESISTANT
Dispense in light-resistant containers
Store at 20º to 25ºC (68º to 77ºF) [See USP Controlled Room Temperature]
USUAL DOSAGE: See package insert
Keep out of reach of children
Manufactured By:
Tulex Pharmaceuticals Inc.
Cranbury, NJ 08512
Distributed By:
TruPharma LLC,
Tampa, FL33609
Code No. LA3801000300 Rev 06/2023

NDC 52817-381-10 Rx Only
Naproxen
Delayed-Release Tablets, USP
500 mg
PHARMACIST: Dispense the Medication Guide provided separately to each patient
100 Tablets
Medication Guide availbale at website www.trupharma.com
TAMPER-EVIDENT CONTAINER.
DO NOT USE IF SEAL IS BROKEN
PACKAGE IS CHILD-RESISTANT
Dispense in light-resistant containers
Store at 20º to 25ºC (68º to 77ºF) [See USP Controlled Room Temperature]
USUAL DOSAGE: See package insert
Keep out of reach of children
Manufactured By:
Tulex Pharmaceuticals Inc.
Cranbury, NJ 08512
Distributed By:
TruPharma LLC
Tampa, FL 33609
Code No. LA3811000300 Rev 06/2023








