NDC Code(s) : 50580-535-01, 50580-535-08
Packager : Johnson & Johnson Consumer Inc.
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Childrens Benadryl DYE-FREE ALLERGYDiphenhydramine hydrochloride SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Johnson & Johnson Consumer Inc.(878046358) |
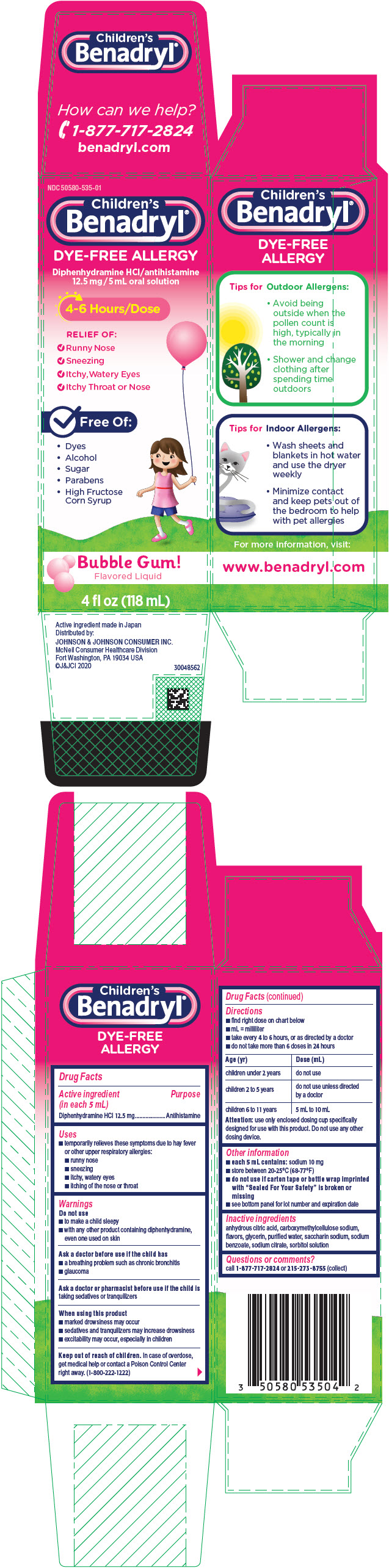
PRINCIPAL DISPLAY PANEL
NDC 50580-535-01
Children's
Benadryl
®
DYE-FREE ALLERGY
Diphenhydramine HCl/antihistamine
12.5 mg/5 mL oral solution
4-6 Hours/Dose
RELIEF OF:
- Runny Nose
- Sneezing
- Itchy,Watery Eyes
- Itchy Throat or Nose
✔ Free Of:
- Dyes
- Alcohol
- Sugar
- Parabens
- High Fructose
Corn Syrup
Bubble Gum!
Flavored Liquid
4 fl oz (118 mL)