NDC Code(s) : 49884-306-02, 49884-307-02, 49884-308-02, 49884-309-02, 49884-310-02
Packager : Par Pharmaceutical, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CIV
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Clonazepamclonazepam TABLET, ORALLY DISINTEGRATING | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Clonazepamclonazepam TABLET, ORALLY DISINTEGRATING | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Clonazepamclonazepam TABLET, ORALLY DISINTEGRATING | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Clonazepamclonazepam TABLET, ORALLY DISINTEGRATING | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Clonazepamclonazepam TABLET, ORALLY DISINTEGRATING | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Par Pharmaceutical, Inc.(092733690) |
| REGISTRANT - Par Pharmaceutical Inc.(092733690) |
PRINCIPAL DISPLAY PANEL
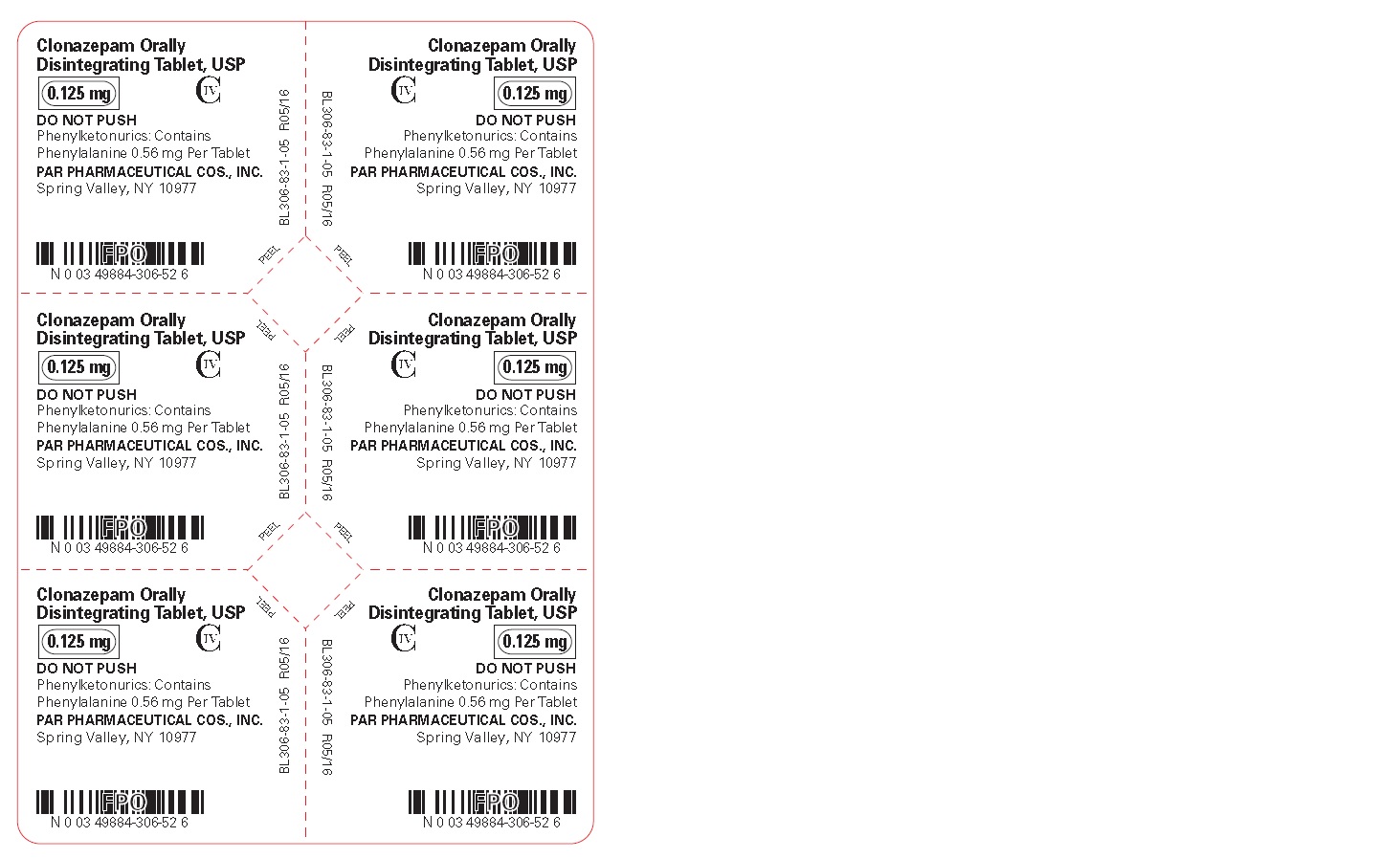 Clonazepam ODT, USP 0.125 mg - Blister
Clonazepam ODT, USP 0.125 mg - Blister
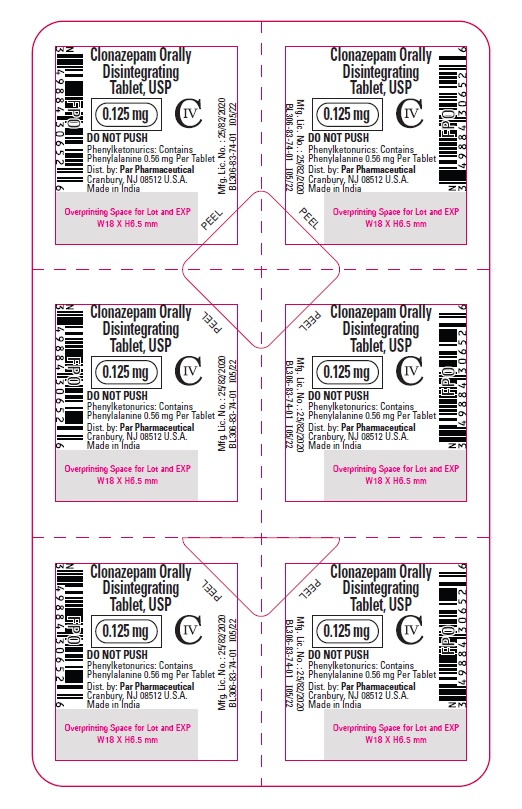 Clonazepam ODT, USP 0.125 mg - Blister
Clonazepam ODT, USP 0.125 mg - Blister
PRINCIPAL DISPLAY PANEL
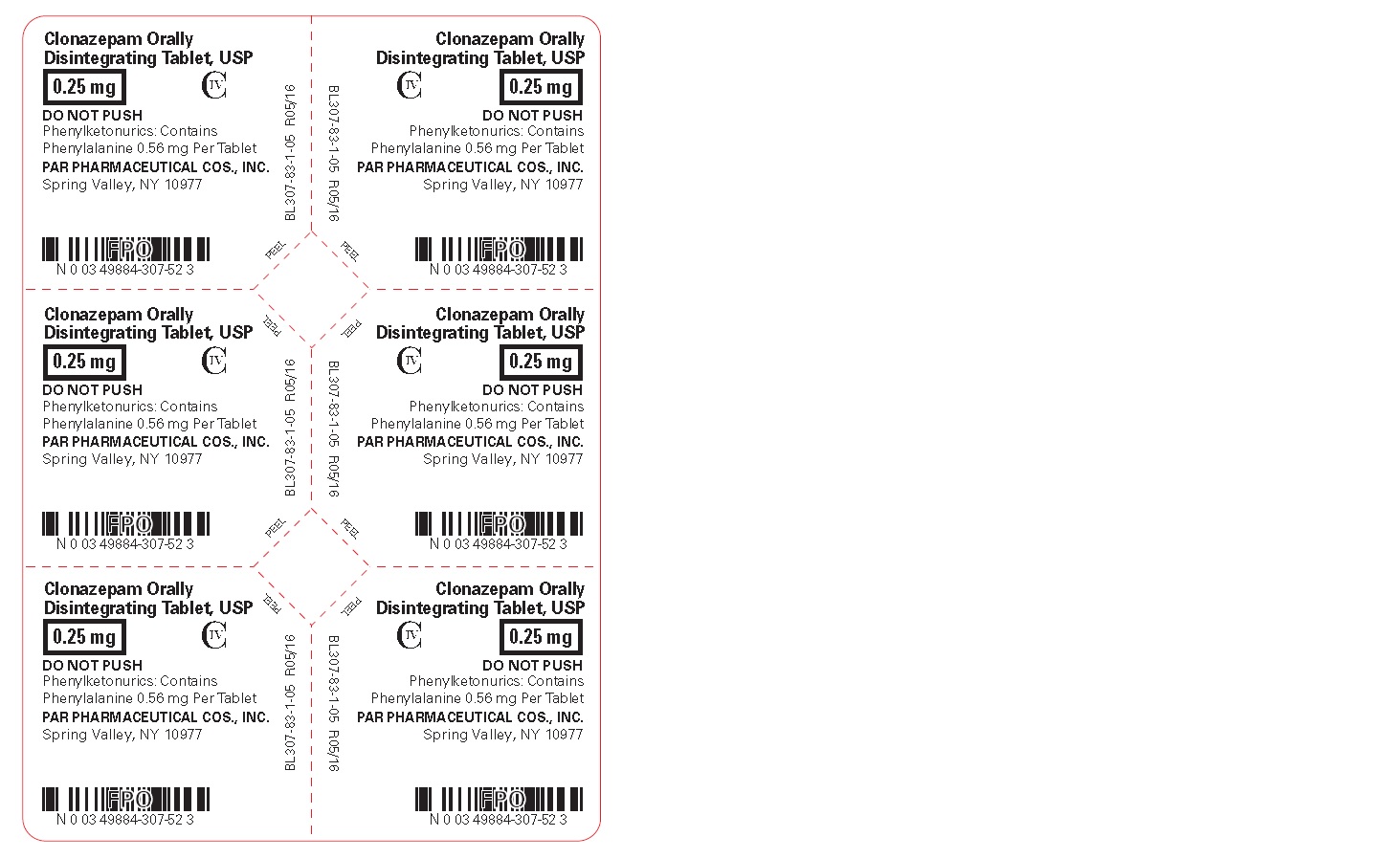 Clonazepam ODT, USP 0.25 mg - Blister
Clonazepam ODT, USP 0.25 mg - Blister
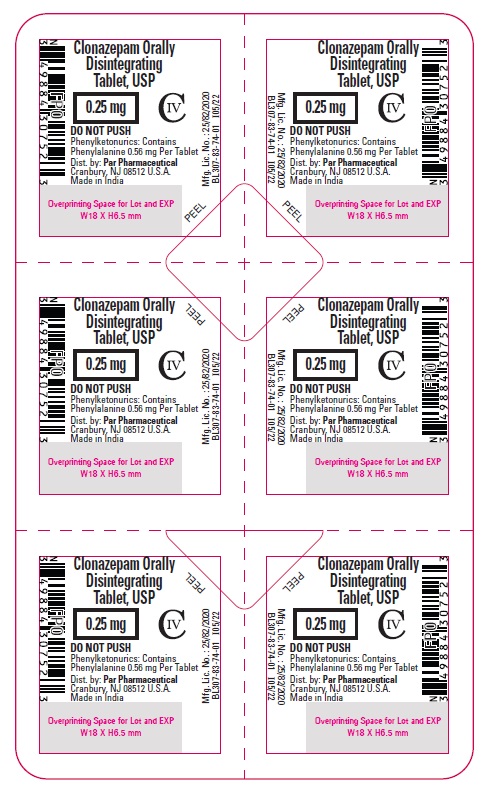 Clonazepam ODT, USP 0.25 mg - Blister
Clonazepam ODT, USP 0.25 mg - Blister
PRINCIPAL DISPLAY PANEL
 Clonazepam ODT, USP 0.5 mg - Blister
Clonazepam ODT, USP 0.5 mg - Blister
PRINCIPAL DISPLAY PANEL
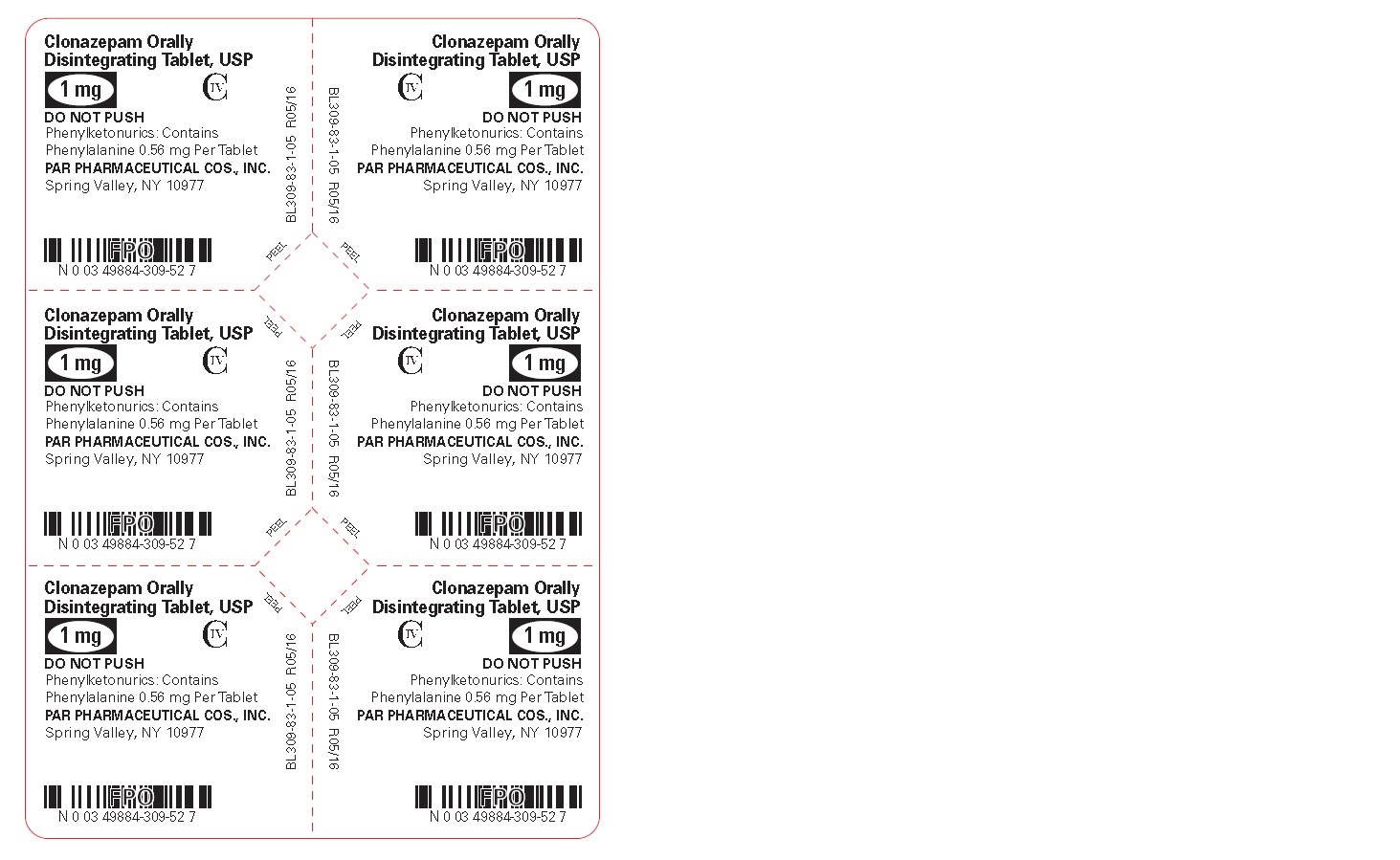 Clonazepam ODT, USP 1 mg - Blister
Clonazepam ODT, USP 1 mg - Blister
PRINCIPAL DISPLAY PANEL
 Clonazepam ODT, USP 2 mg - Blister
Clonazepam ODT, USP 2 mg - Blister
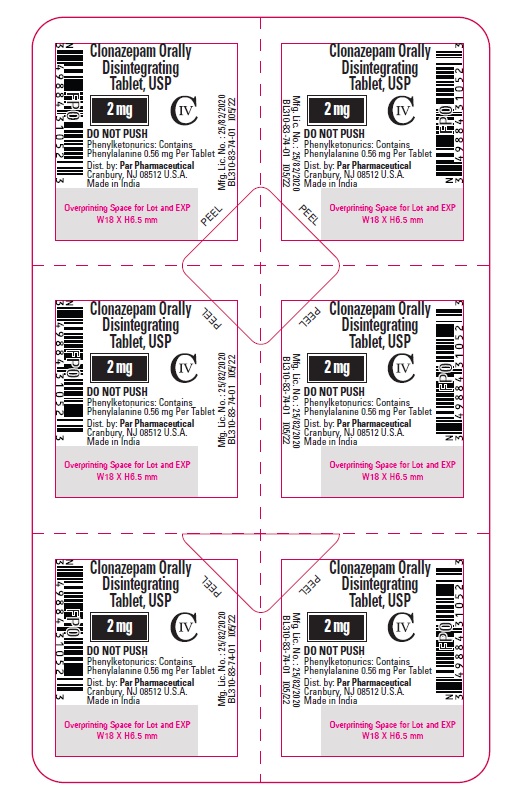 Clonazepam ODT, USP 2 mg - Blister
Clonazepam ODT, USP 2 mg - Blister
PRINCIPAL DISPLAY PANEL

 0.125 mg carton
0.125 mg carton
PRINCIPAL DISPLAY PANEL

 0.25 mg carton.
0.25 mg carton.
PRINCIPAL DISPLAY PANEL

PRINCIPAL DISPLAY PANEL

PRINCIPAL DISPLAY PANEL

 2 mg carton
2 mg carton









