NDC Code(s) : 49527-051-01, 49527-051-02, 49527-051-03, 49527-051-04, 49527-051-05
Packager : CLINIQUE LABORATORIES LLC
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CLINIQUE SMART BROAD SPECTRUM SPF 15 CUSTOM-REPAIR MOISTURIZER DRY COMBINATIONavobenzone and octisalate CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - CLINIQUE LABORATORIES LLC(044475127) |
| REGISTRANT - Estee Lauder Companies Inc.(790802086) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Estee Lauder Cosmetics Ltd. | 202952982 | manufacture(49527-051) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Estee Lauder Cosmetics Ltd. | 204132062 | pack(49527-051), label(49527-051) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| The Estee Lauder Inc | 802599436 | manufacture(49527-051), pack(49527-051), label(49527-051) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Northtec LLC | 943871157 | pack(49527-051), label(49527-051) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| NORTHTEC KEYSTONE | 949264774 | pack(49527-051), label(49527-051) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| PALC | 078364654 | pack(49527-051), label(49527-051) | |
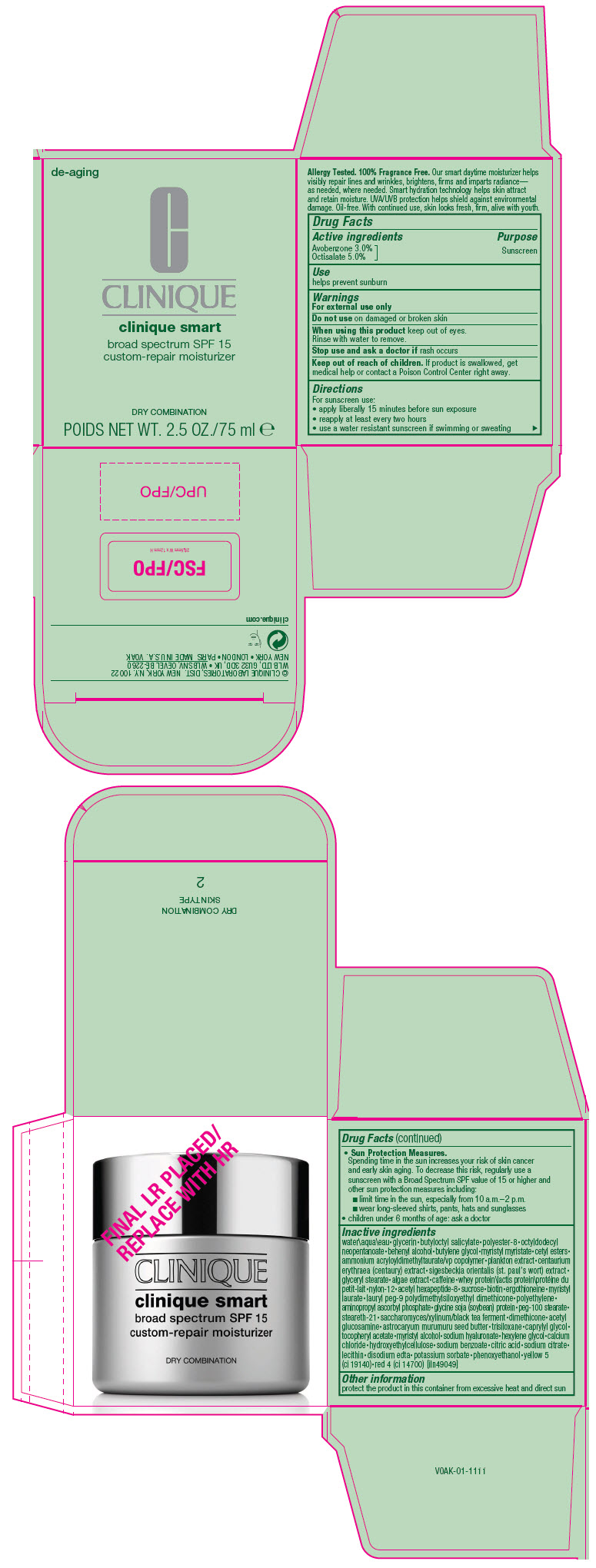
PRINCIPAL DISPLAY PANEL
de-aging
CLINIQUE
clinique smart
broad spectrum SPF 15
custom-repair moisturizer
DRY COMBINATION
POIDS NET WT. 2.5 OZ./75 ml ℮