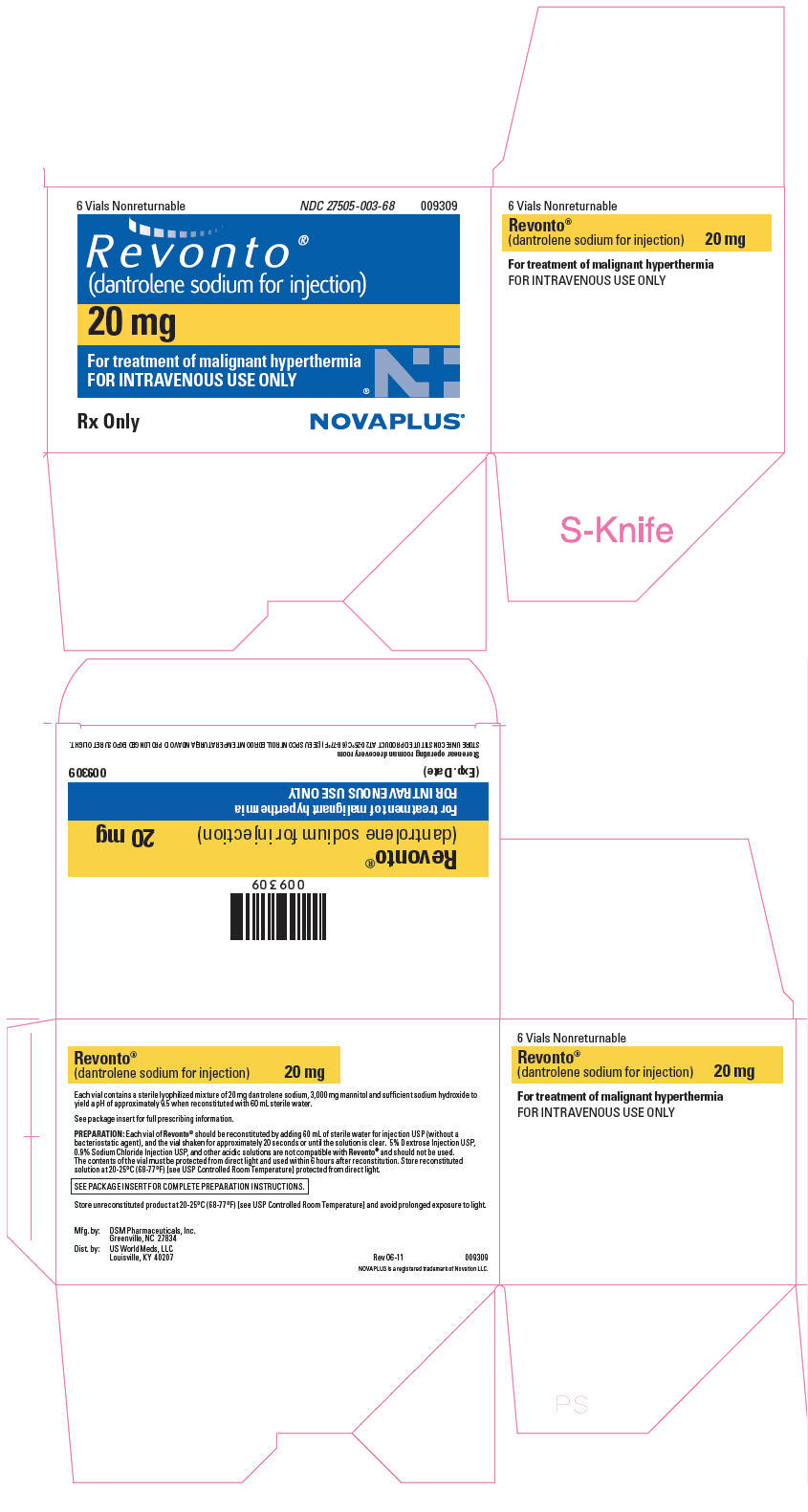
NDC Code(s) : 27505-003-68
Packager : US WorldMeds, LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Revontodantrolene sodium INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
6 Vials Nonreturnable
NDC 27505-003-68
009309
Revonto®
(dantrolene sodium for injection)
20 mg
For treatment of malignant hyperthermia
FOR INTRAVENOUS USE ONLY
Rx Only
NOVAPLUS
®