NDC Code(s) : 23155-836-01, 23155-836-05
Packager : Avet Pharmaceuticals Inc
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Mycophenolate mofetil Mycophenolate mofetil TABLET, FILM COATED | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| LABELER - Avet Pharmaceuticals Inc(780779901) |
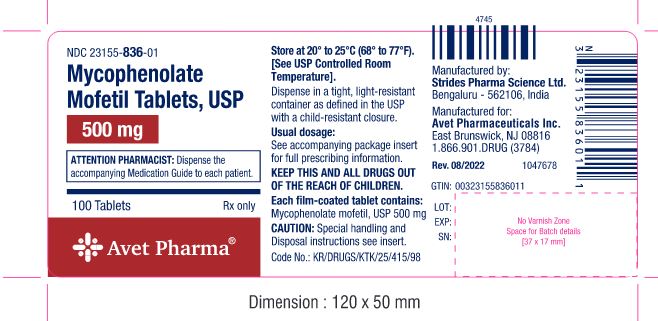
PRINCIPAL DISPLAY PANEL
Mycophenolate Mofetil Tablets, USP
500 mg
Rx Only
100 Tablets
NDC 21355-836-01
Mycophenolate Mofetil Tablets, USP
500 mg
Rx Only
500 Tablets
NDC 21355-836-05










