NDC Code(s) : 16477-505-01, 16477-505-21, 16477-505-48
Packager : Laser Pharmaceuticals, LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| MILLIPREDPREDNISOLONE TABLET | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
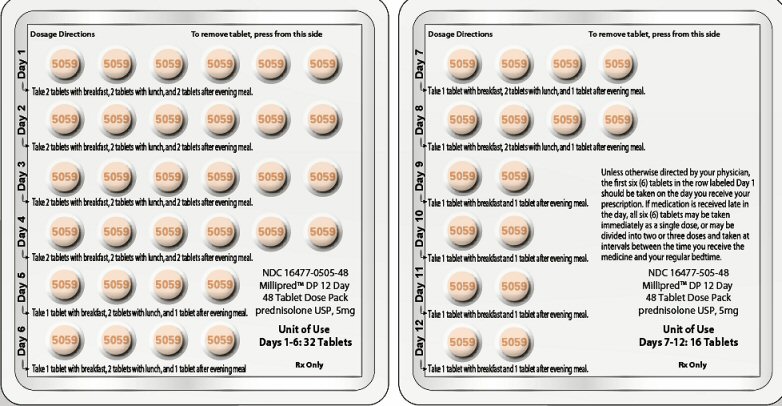
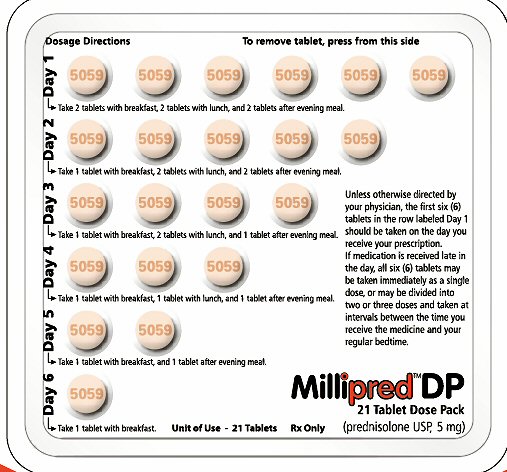
PRINCIPAL DISPLAY PANEL
MANUFACTURED BY: WATSON PHARMA PRIVATE LIMITED VERNA, SALCETTE Goa 403 722 INDIA
DISTRIBUTED BY: LASER PHARMACEUTICALS, LLC GREENVILLE, SC 29615
REVISED : 8/2011 0811A 173415