NDC Code(s) : 10019-955-50, 10019-955-01, 10019-956-16, 10019-956-01, 10019-957-11, 10019-957-01
Packager : Baxter Healthcare Corporation
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Cyclophosphamidecyclophosphamide INJECTION, POWDER, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Cyclophosphamidecyclophosphamide INJECTION, POWDER, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Cyclophosphamidecyclophosphamide INJECTION, POWDER, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Baxter Healthcare Corporation(005083209) |
| REGISTRANT - Baxter Healthcare Corporation(005083209) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Baxter Oncology GmbH | 344276063 | ANALYSIS(10019-955, 10019-956, 10019-957), LABEL(10019-955, 10019-956, 10019-957), MANUFACTURE(10019-955, 10019-956, 10019-957), PACK(10019-955, 10019-956, 10019-957), STERILIZE(10019-955, 10019-956, 10019-957), API MANUFACTURE(10019-955, 10019-956, 10019-957) | |
PRINCIPAL DISPLAY PANEL
 Container Label
Container Label
LOT/EXP:
NDC 10019-955-50
Cyclophosphamide
for Injection, USP
500 mg/vial CYTOTOXIC AGENT
FOR SINGLE DOSE USE
STERILE, NON-PYROGENIC
FOR PARENTERAL USE
Rx only
Baxter Logo
Manufactured by Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Made in Germany
460-629-01
Each vial contains 500 mg cyclophosphamide.
For IV Infusion Use: Add 25 mL Sterile
Water for Injection, USP, and shake
vigorously to dissolve the drug.
For Direct Injection Use: Add 25 mL
0.9% Sodium Chloride Injection, USP,
and shake vigorously to dissolve
the drug.
See insert for indications and dosage
schedule. Store vial at or below 25˚C
(77˚F) [see USP Controlled Room
Temperature].
Bar Code
(01)00310019955506
USA HA-65-01-628 C 273
Bar Code

 Carton Label
Carton Label
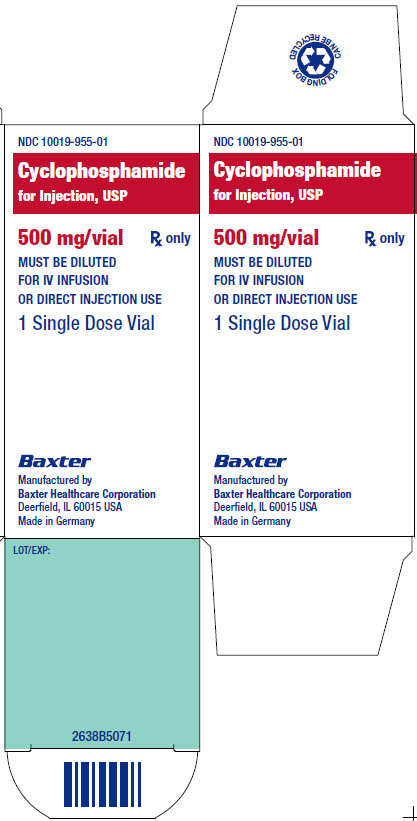
NDC 10019-955-01
Cyclophosphamide
for Injection, USP
500 mg/vial
Rx only
MUST BE DILUTED
FOR IV INFUSION
OR DIRECT INJECTION USE
1 Single Dose Vial
Baxter Logo
Manufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Made in Germany
Cyclophosphamide
for Injection, USP
500 mg/vial
Bar Code
HA-80-02-313
USA
Each vial contains 500 mg cyclophosphamide.
For IV Infusion Use: Add 25 mL Sterile Water
for Injection, USP, and shake vigorously to
dissolve the drug.
For Direct Injection Use: Add 25 mL 0.9%
Sodium Chloride Injection, USP, and shake
vigorously to dissolve the drug.
Store vial at or below 25°C (77°F)
[see USP Controlled Room Temperature].
DOSAGE: See accompanying literature for
directions for use. Should not be prescribed
without thorough knowledge of dose,
indications and toxicity as contained in
accompanying literature.
CYTOTOXIC AGENT
FOR SINGLE DOSE USE
STERILE, NON-PYROGENIC
FOR PARENTERAL USE
460-630-01
C
628
NDC 10019-955-01
Cyclophosphamide
for Injection, USP
500 mg/vial
Rx only
MUST BE DILUTED
FOR IV INFUSION
OR DIRECT INJECTION USE
1 Single Dose Vial
Baxter Logo
Manufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Made in Germany
LOT /EXP:
2638B5071
Barcode
FOLDING BOX
CAN BE RECYCLED Symbol
NDC 10019-955-01
Cyclophosphamide
for Injection, USP
500 mg/vial
Rx only
MUST BE DILUTED
FOR IV INFUSION
OR DIRECT INJECTION USE
1 Single Dose Vial
Baxter Logo
Manufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Made in Germany
 Container Label
Container Label
LOT/EXP:
NDC 10019-956-16
Cyclophosphamide
for Injection, USP
1 gram/vial CYTOTOXIC AGENT
FOR SINGLE DOSE USE
STERILE, NON-PYROGENIC
FOR PARENTERAL USE
Rx only
Baxter Logo
Manufactured by Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Made in Germany
460-631-01
Each vial contains 1 g cyclophosphamide.
For IV Infusion Use: Add 50 mL Sterile
Water for Injection, USP, and shake
vigorously to dissolve the drug.
For Direct Injection Use: Add 50 mL
0.9% Sodium Chloride Injection,
USP, and shake vigorously to dissolve
the drug.
See insert for indications and dosage
schedule. Store vial at or below 25˚C
(77˚F) [see USP Controlled Room
Temperature].
Bar Code
(01)00310019956169
USA HA-65-01-629
C 270
Bar Code
 Carton Label
Carton Label
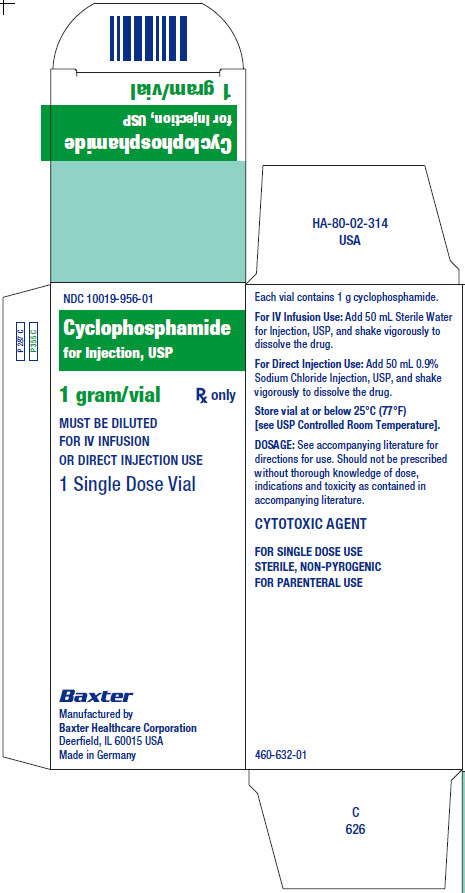
NDC 10019-956-01
Cyclophosphamide
for Injection, USP
1 gram/vial
Rx only
MUST BE DILUTED
FOR IV INFUSION
OR DIRECT INJECTION USE
1 Single Dose Vial
Baxter Logo
Manufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Made in Germany
Cyclophosphamide
for Injection, USP
1 gram/vial
HA-80-02-314
USA
Each vial contains 1 g cyclophosphamide.
For IV Infusion Use: Add 50 mL Sterile Water
for Injection, USP, and shake vigorously to
dissolve the drug.
For Direct Injection Use: Add 50 mL 0.9%
Sodium Chloride Injection, USP, and shake
vigorously to dissolve the drug.
Store vial at or below 25°C 77°F)
[see USP Controlled Room Temperature].
DOSAGE: See accompanying literature for
directions for use. Should not be prescribed
without thorough knowledge of dose,
indications and toxicity as contained in
accompanying literature.
CYTOTOXIC AGENT
FOR SINGLE DOSE USE
STERILE, NON-PYROGENIC
FOR PARENTERAL USE
460-632-01
C
626
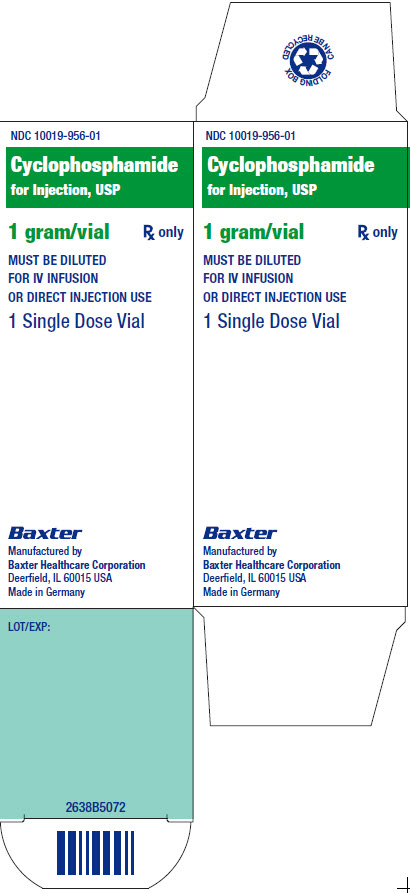
NDC 10019-956-01
Cyclophosphamide
for Injection, USP
1 gram/vial Rx only
MUST BE DILUTED
FOR IV INFUSIONOR
DIRECT INJECTION USE
1 Single Dose Vial
Baxter Logo
Manufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Made in Germany
LOT /EXP:
2638B5072
Barcode
FOLDING BOX
CAN BE RECYCLED Symbol
NDC 10019-956-01
Cyclophosphamide
for Injection, USP
1 gram/vial
Rx only
MUST BE DILUTED
FOR IV INFUSIONOR
DIRECT INJECTION USE
1 Single Dose Vial
Baxter Logo
Manufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Made in Germany
 Container Label
Container Label
LOT/EXP:
NDC 10019-957-11
Cyclophosphamide
for Injection, USP
2 gram/vial CYTOTOXIC AGENT
FOR SINGLE DOSE USE
STERILE, NON-PYROGENIC
FOR PARENTERAL USE
Rx only
Baxter Logo
Manufactured by Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Made in Germany
460-633-01
Each vial contains 2 g cyclophosphamide.
For IV Infusion Use: Add 100 mL
Sterile Water for Injection, USP, and
shake vigorously to dissolve the drug.
For Direct Injection Use: Add 100 mL
0.9% Sodium Chloride Injection, USP,
and shake vigorously to dissolve
the drug.
See insert for indications and dosage
schedule. Store vial at or below 25˚C
(77˚F) [see USP Controlled Room
Temperature].
Barcode
(01)00310019957111
USA HA-65-01-630
C271

 Carton Label
Carton Label
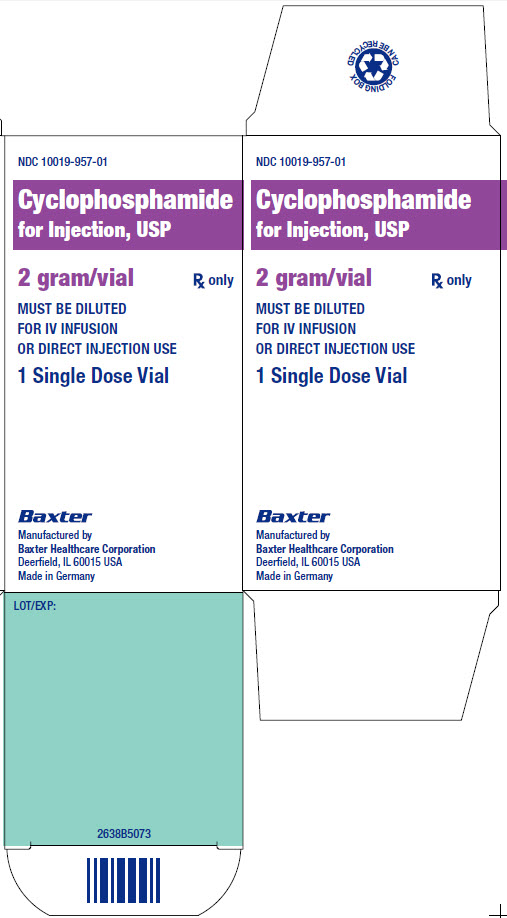
NDC 10019-957-01
Cyclophosphamide
for Injection, USP
2 gram/vial
Rx only
MUST BE DILUTED
FOR IV INFUSION
OR DIRECT INJECTION USE
1 Single Dose Vial
BaxterLogo
Manufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Made in Germany
Cyclophosphamide
for Injection, USP
2 gram/vial
Bar Code
HA-80-02-315
USA
Each vial contains 2 g cyclophosphamide.
For IV Infusion Use: Add 100 mL Sterile
Water for Injection, USP, and shake vigorously to
dissolve the drug.
For Direct Injection Use: Add 100 mL
0.9% Sodium Chloride Injection, USP, and
shake vigorously to dissolve the drug.
Store vial at or below 25°C 77°F)
[see USP Controlled Room Temperature].
DOSAGE: See accompanying literature
for directions for use. Should not be prescribed
without thorough knowledge of dose,
indications and toxicity as contained in
accompanying literature.
CYTOTOXIC AGENT
FOR SINGLE DOSE USE
STERILE, NON-PYROGENIC
FOR PARENTERAL USE
460-634-01
C
627
NDC 10019-957-01
Cyclophosphamide
for Injection, USP
2 gram/vial
Rx only
MUST BE DILUTED
FOR IV INFUSIONOR
DIRECT INJECTION USE
1 Single Dose Vial
Baxter Logo
Manufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Made in Germany
LOT /EXP:
2638B5073
Bar Code
NDC 10019-957-01
Cyclophosphamide
for Injection, USP
2 gram/vial
Rx only
MUST BE DILUTED
FOR IV INFUSIONOR
DIRECT INJECTION USE
1 Single Dose Vial
Baxter Logo
Manufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Made in Germany
FOLDING BOX
CAN BE RECYCLED Symbol