NDC Code(s) : 0378-8170-91, 0378-8171-91, 0378-8172-91
Packager : Mylan Pharmaceuticals Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CIII
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| DronabinolDronabinol CAPSULE, LIQUID FILLED | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| DronabinolDronabinol CAPSULE, LIQUID FILLED | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| DronabinolDronabinol CAPSULE, LIQUID FILLED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
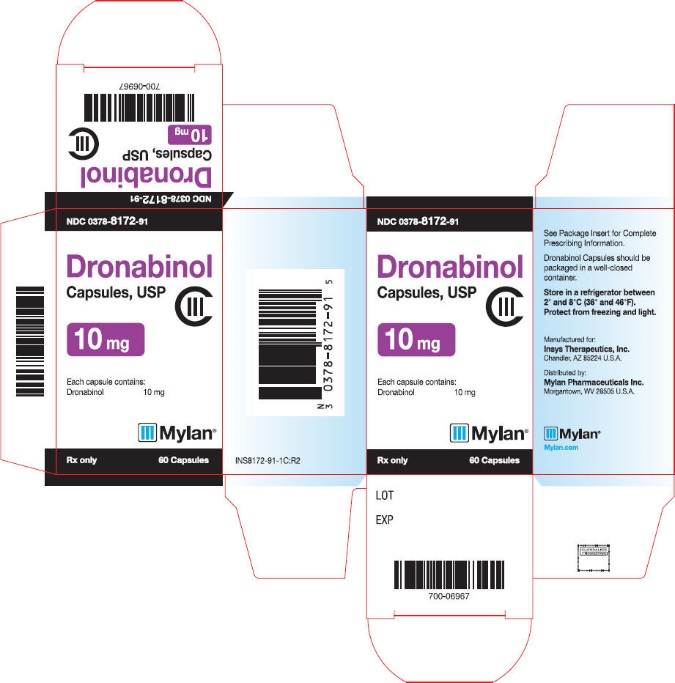
PRINCIPAL DISPLAY PANEL
NDC 0378-8170-91
Dronabinol
Capsules, USP
CIII
2.5 mg
Each capsule contains:
Dronabinol 2.5 mg
Mylan®
Rx only
60 Capsules
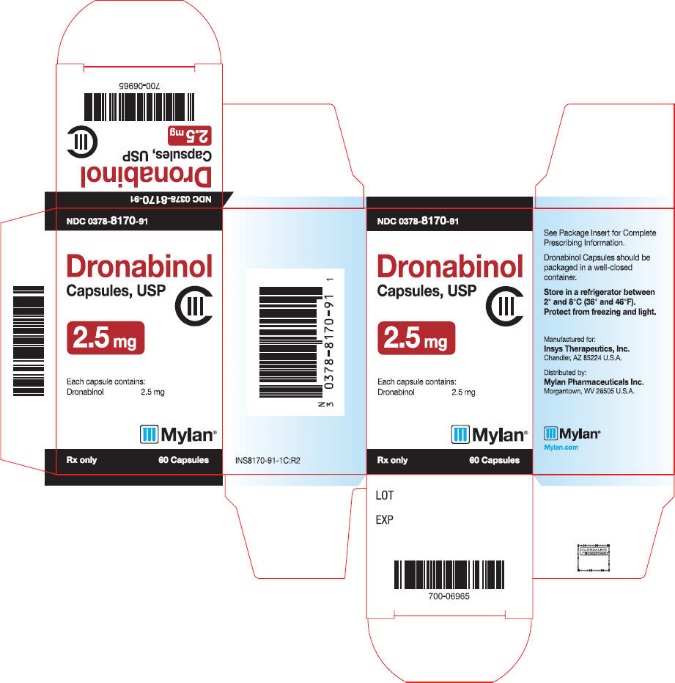
PRINCIPAL DISPLAY PANEL
NDC 0378-8171-91
Dronabinol
Capsules, USP
CIII
5 mg
Each capsule contains:
Dronabinol 5 mg
Mylan®
Rx only
60 Capsules
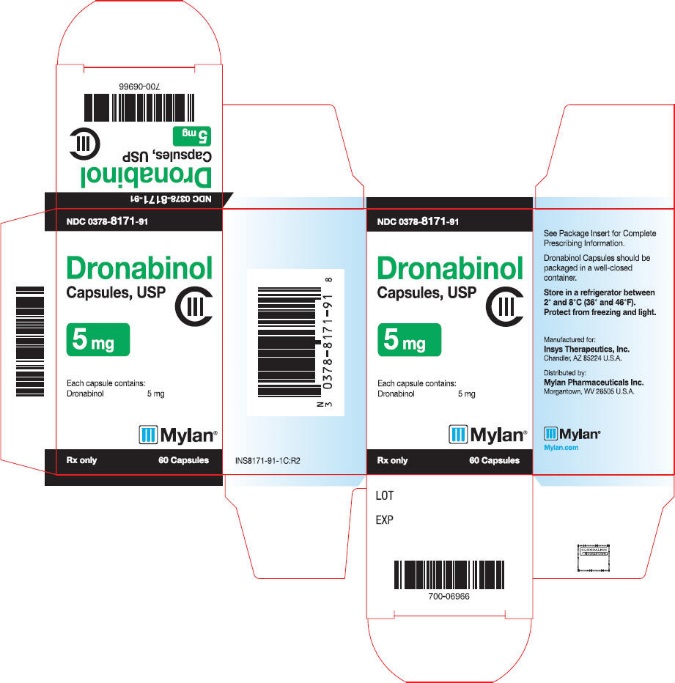
PRINCIPAL DISPLAY PANEL
NDC 0378-8172-91
Dronabinol
Capsules, USP
CIII
10 mg
Each capsule contains:
Dronabinol 10 mg
Mylan®
Rx only
60 Capsules