NDC Code(s) : 0378-0630-91, 0378-0630-01, 0378-0630-88, 0378-0631-91, 0378-0631-01, 0378-0631-88, 0378-0632-91, 0378-0632-01, 0378-0632-88, 0378-0633-91, 0378-0633-01, 0378-0633-88, 0378-0634-91, 0378-0634-01, 0378-0634-88, 0378-0635-91, 0378-0635-01, 0378-0635-88, 0378-0636-91, 0378-0636-01, 0378-0636-88
Packager : Mylan Pharmaceuticals Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| IloperidoneIloperidone TABLET | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| IloperidoneIloperidone TABLET | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| IloperidoneIloperidone TABLET | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| IloperidoneIloperidone TABLET | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| IloperidoneIloperidone TABLET | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| IloperidoneIloperidone TABLET | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| IloperidoneIloperidone TABLET | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Mylan Pharmaceuticals Inc.(059295980) |
| REGISTRANT - Inventia Healthcare Private Limited(650452522) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Inventia healthcare Limited | 677604412 | ANALYSIS(0378-0630, 0378-0631, 0378-0632, 0378-0633, 0378-0634, 0378-0635, 0378-0636), MANUFACTURE(0378-0630, 0378-0631, 0378-0632, 0378-0633, 0378-0634, 0378-0635, 0378-0636) | |
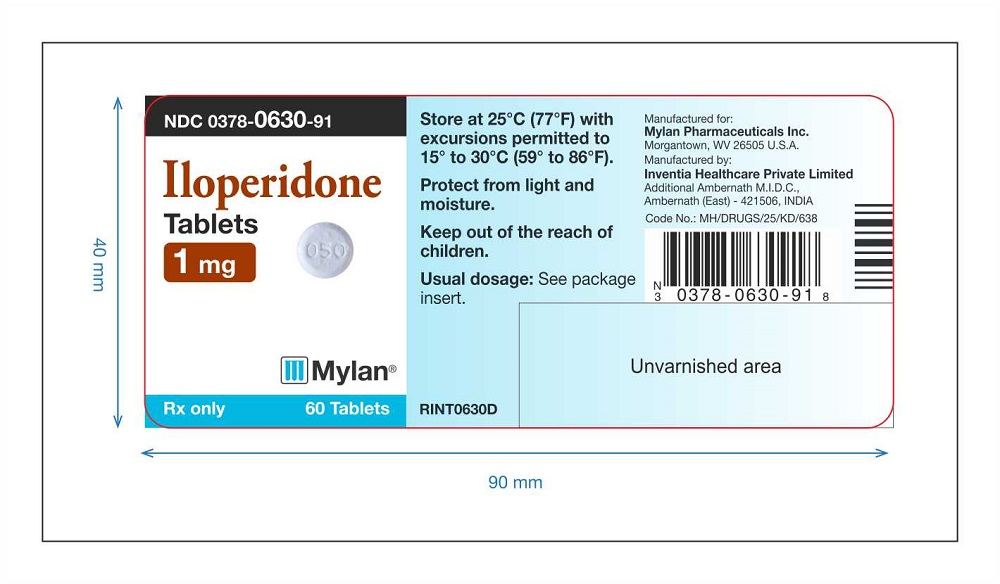
PRINCIPAL DISPLAY PANEL
Iloperidone Tablets 1 mg Label 60 counts
PRINCIPAL DISPLAY PANEL
Iloperidone Tablets 2 mg Label 60 counts

PRINCIPAL DISPLAY PANEL
Iloperidone Tablets 4 mg Label 60 counts

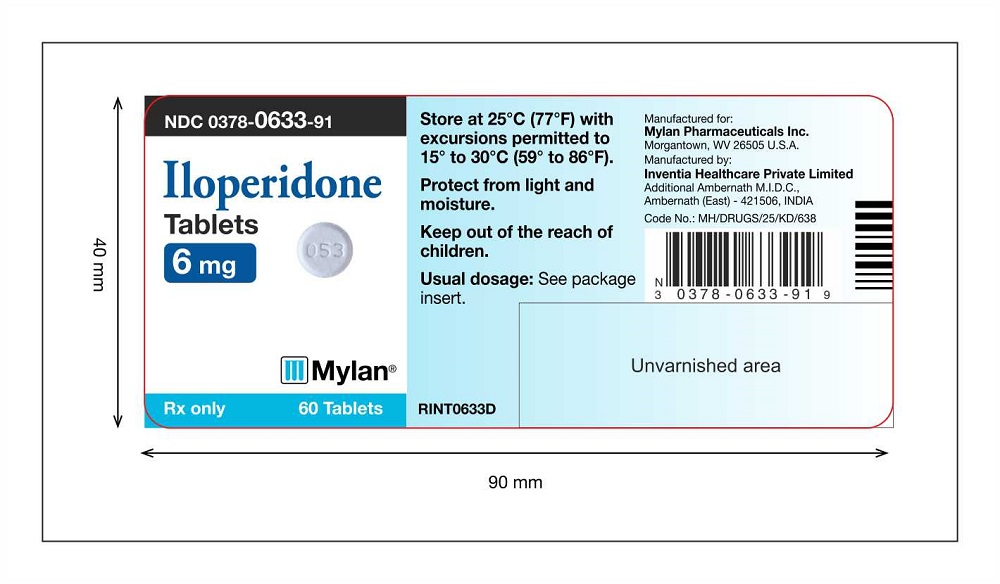
PRINCIPAL DISPLAY PANEL
Iloperidone Tablets 6 mg Label 60 counts
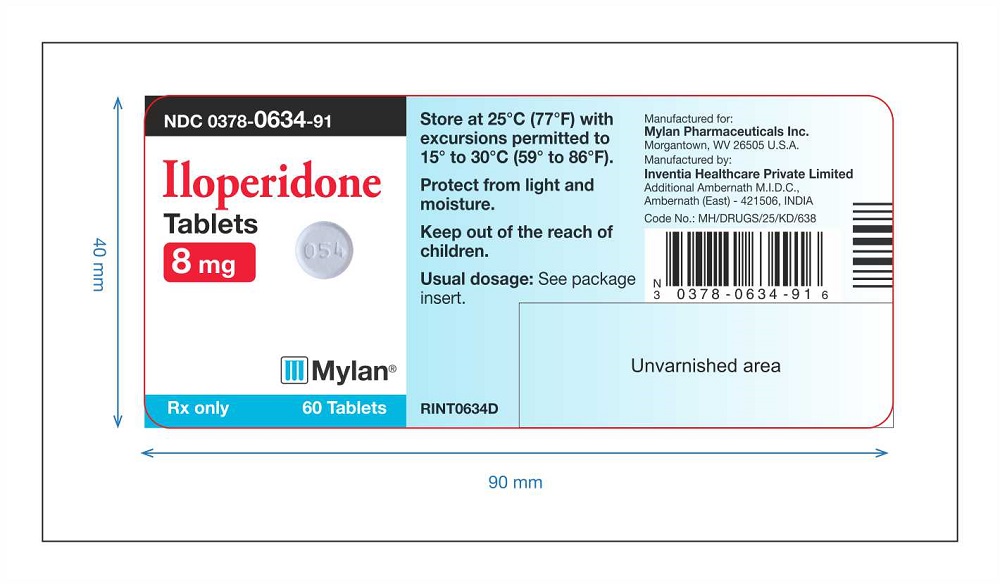
PRINCIPAL DISPLAY PANEL
Iloperidone Tablets 8 mg Label 60 counts
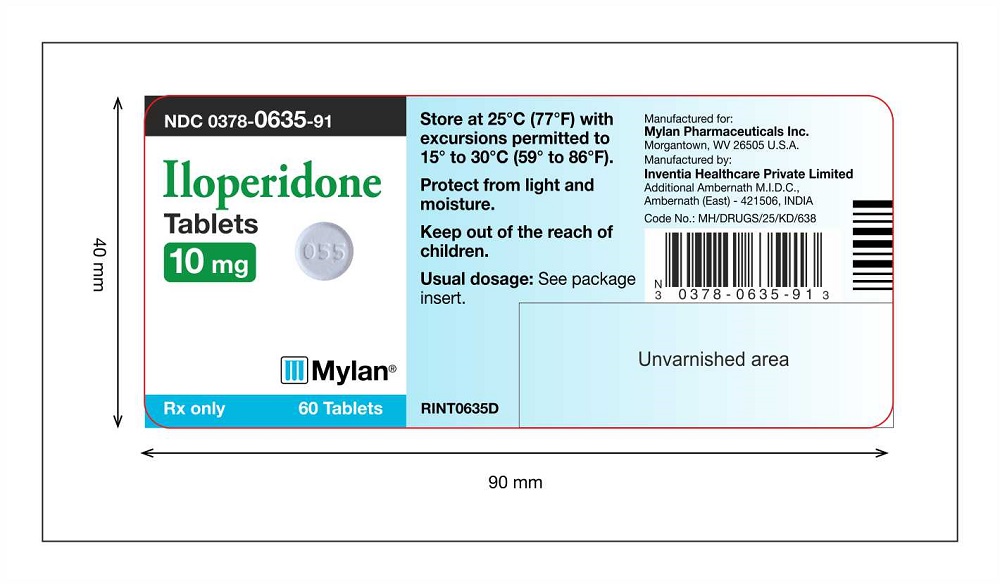
PRINCIPAL DISPLAY PANEL
Iloperidone Tablets 10 mg Label 60 counts
PRINCIPAL DISPLAY PANEL
Iloperidone Tablets 12 mg Label 60 counts










