NDC Code(s) : 0186-0510-60, 0186-0520-06, 0186-0520-60, 0186-0520-39
Packager : AstraZeneca LP
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| VIMOVOnaproxen and esomeprazole magnesium TABLET, DELAYED RELEASE | ||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| VIMOVOnaproxen and esomeprazole magnesium TABLET, DELAYED RELEASE | ||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
NDC 0186-0510-60
60 tablets
VIMOVO®
(naproxen and esomeprazole magnesium)
Delayed release tablets
375 mg/20 mg*
*Each tablet contains 22.3 mg esomeprazole magnesium,
Equivalent to 20 mg of esomeprazole.
Dispense the enclosed Medication Guide to each patient.
Rx only
AstraZeneca

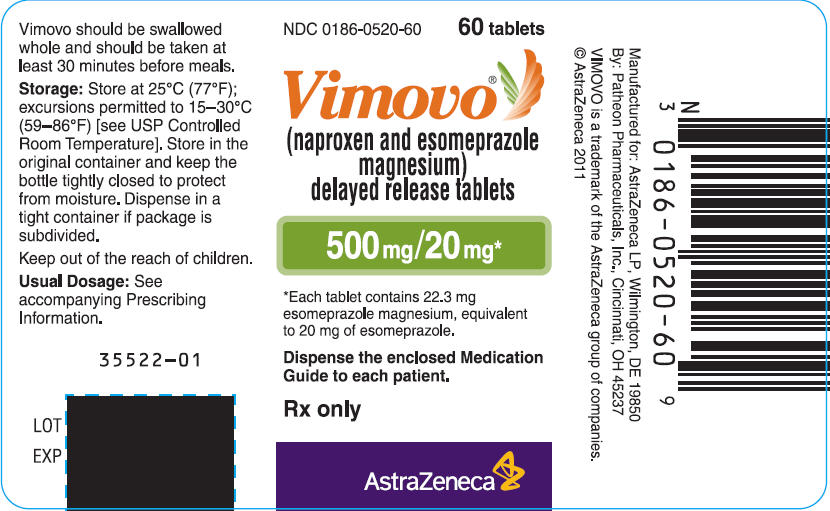
PRINCIPAL DISPLAY PANEL
NDC 0186-0520-60
60 tablets
VIMOVO®
(naproxen and esomeprazole magnesium)
Delayed release tablets
500 mg/20 mg*
*Each tablet contains 22.3 mg esomeprazole magnesium,
Equivalent to 20 mg of esomeprazole.
Dispense the enclosed Medication Guide to each patient.
Rx only
AstraZeneca