NDC Code(s) : 0169-7501-11, 0169-7501-90, 0169-3303-12, 0169-3303-91, 0169-3303-90, 0169-6339-10, 0169-6339-90, 0169-6339-97, 0169-6339-98, 0169-6338-10, 0169-6338-90, 0169-6338-98, 0169-2001-90, 0169-2100-11, 0169-2101-12, 0169-2101-25
Packager : Novo Nordisk
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| NOVOLOGinsulin aspart INJECTION, SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| NOVOLOGinsulin aspart INJECTION, SOLUTION | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| NOVOLOGinsulin aspart INJECTION, SOLUTION | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| NOVOLOGinsulin aspart INJECTION, SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Insulin Diluting Medium for NovoLogwater injection INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| NOVOLOGinsulin aspart INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| NOVOLOGinsulin aspart INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Novo Nordisk(622920320) |
PRINCIPAL DISPLAY PANEL
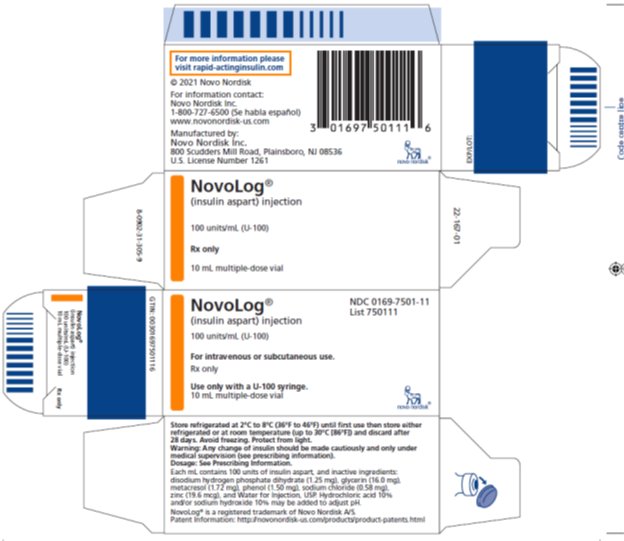
- NovoLog®NDC 0169-7501-11
- (insulin aspart) injection List 750111
100 units/mL (U-100)
For intravenous or subcutaneous use.
Rx only
Use only with a U-100 syringe.
10 mL multiple-dose vial
PRINCIPAL DISPLAY PANEL
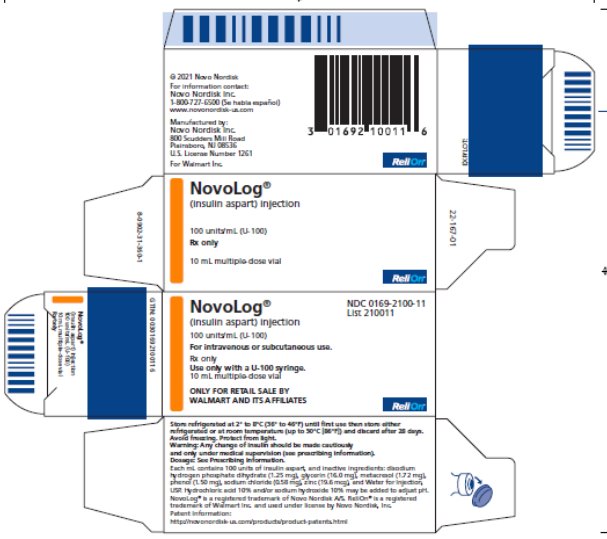
NovoLog®
- (insulin aspart) injection
NDC 0169-2100-11
List 210011
100 units/mL (U-100)
For intravenous or subcutaneous use.
Rx only
Use only with a U-100 syringe.
10 mL multiple-dose vial
ONLY FOR RETAIL SALE BY WALMART AND ITS AFFILIATES
Relion
PRINCIPAL DISPLAY PANEL
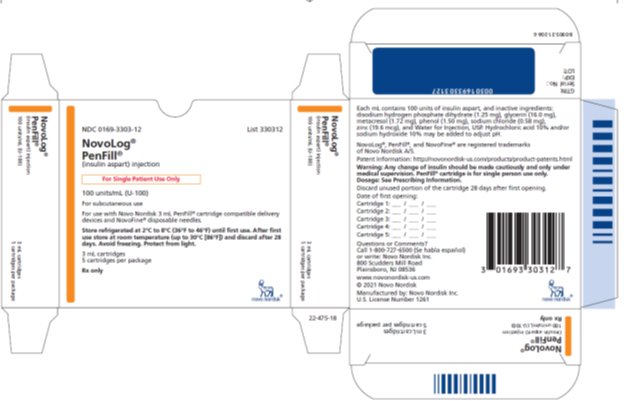
NDC 0169-3303-12 List 330312
NovoLog®
PenFill®
(insulin aspart) injection
For Single Patient Use Only
100 units/mL (U-100)
For subcutaneous use
For use with Novo Nordisk 3 mL PenFill® cartridge compatible delivery devices and NovoFine® disposable needles.
Store refrigerated at 2°C to 8°C (36°F to 46°F) until first use. After first use store at room temperature (up to 30°C [86°F]) and discard after 28 days. Avoid freezing. Protect from light.
3 mL cartridges
5 cartridges per package
Rx only
PRINCIPAL DISPLAY PANEL
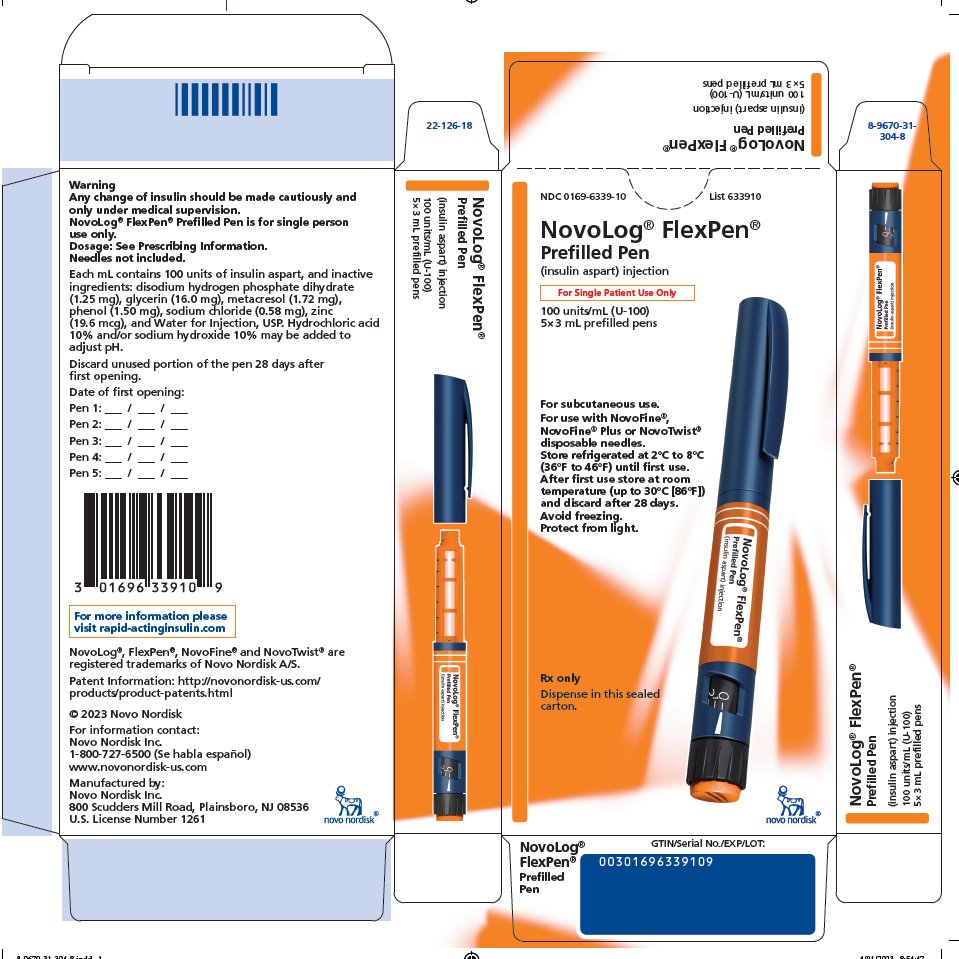
NDC 0169-6339-10 List 633910
NovoLog® FlexPen®
Prefilled Pen
(insulin aspart) injection
For Single Patient Use Only
100 units/mL (U-100)
5 x 3 mL prefilled pens
For subcutaneous use.
For use with NovoFine®, NovoFine® Plus or NovoTwist® disposable needles.
Store refrigerated at 2°C to 8°C (36°F to 46°F) until first use. After first use store at room temperature (up to 30°C [86°F]) and discard after 28 days. Avoid freezing. Protect from light.
Rx only
Dispense in this sealed carton.
PRINCIPAL DISPLAY PANEL
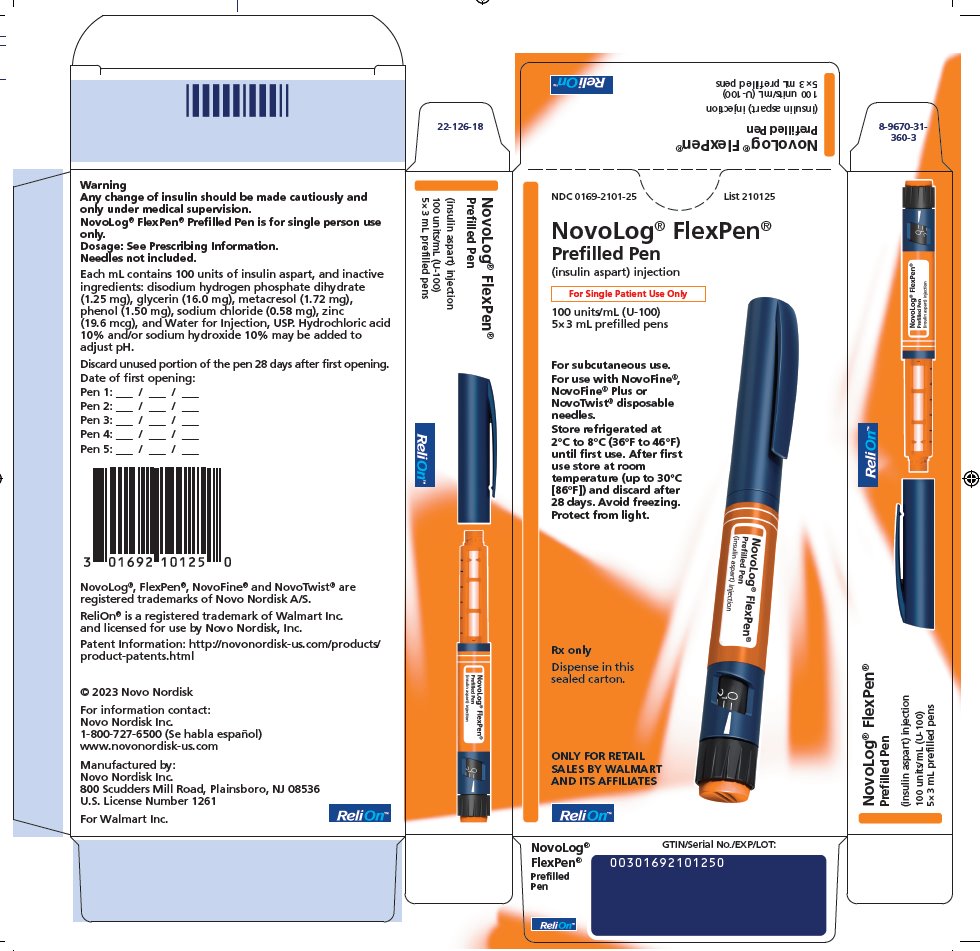
NDC 0169-2101-25
List 210125
NovoLog® FlexPen®
Prefilled pen
(insulin aspart) injection
For Single Patient Use Only
100 units/mL (U-100)
5×3 mL prefilled pens
For subcutaneous use.
For use with NovoFine®, NovoFine® Plus or NovoTwist®
disposable needles.
Store refrigerated at 2°C to 8°C (36°F to 46°F) until first use. After first use store at room temperature (up to 30°C [86°F]) and discard after 28 days. Avoid freezing. Protect from light.
Rx only
Dispense in this sealed carton.
ONLY FOR RETAIL SALES BY WALMART AND ITS AFFILIATES
ReliOn
PRINCIPAL DISPLAY PANEL
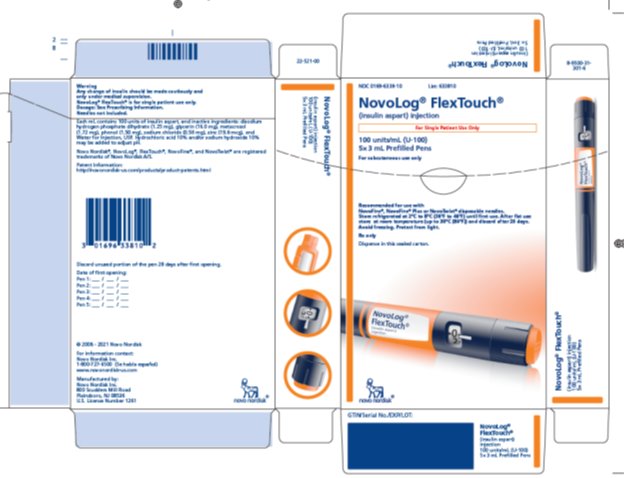
NDC 0169-6338-10 List 633810
NovoLog® FlexTouch®
(insulin aspart) injection
For Single Patient Use Only
100 units/mL (U-100)
5 x 3 mL Prefilled Pens
For subcutaneous use only
Recommended for use with
NovoFine®, NovoFine® Plusor NovoTwist® disposable needles.
Store refrigerated at 2°C to 8°C (36°F to 46°F) until first use. After first use store at room temperature (up to 30°C [86°F]) and discard after 28 days. Avoid freezing. Protect from light.
Rx only
Dispense in this sealed carton.
PRINCIPAL DISPLAY PANEL
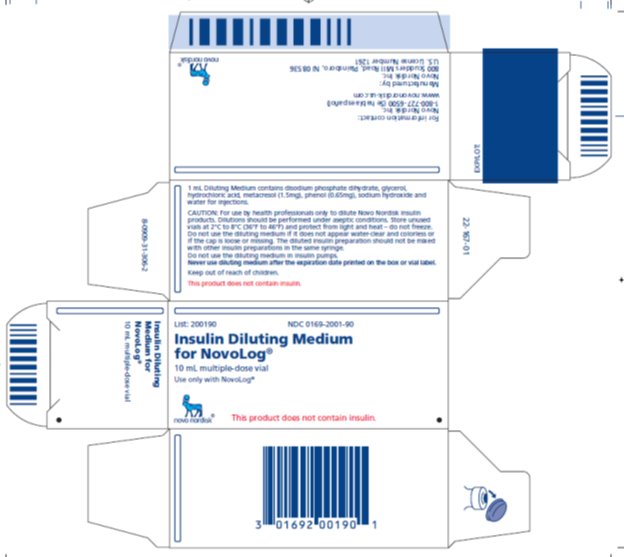
List: 200190 NDC 0169-2001-90
Insulin Diluting Medium
for NovoLog®
10 mL multiple-dose vial
Use only with NovoLog®
This product does not contain insulin.