NDC Code(s) : 0169-4060-13, 0169-4060-12, 0169-4060-90, 0169-4060-97, 0169-4060-98, 0169-4060-99
Packager : Novo Nordisk
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Victozaliraglutide INJECTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - Novo Nordisk(622920320) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Novo Nordisk A/S | 305914798 | MANUFACTURE(0169-4060) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Novo Nordisk Pharmaceutical Industries, LP | 622920320 | MANUFACTURE(0169-4060) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Novo Nordisk A/S | 305156788 | API MANUFACTURE(0169-4060) | |
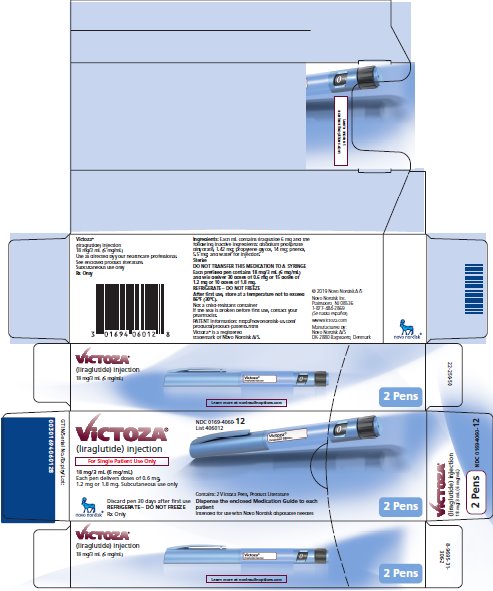
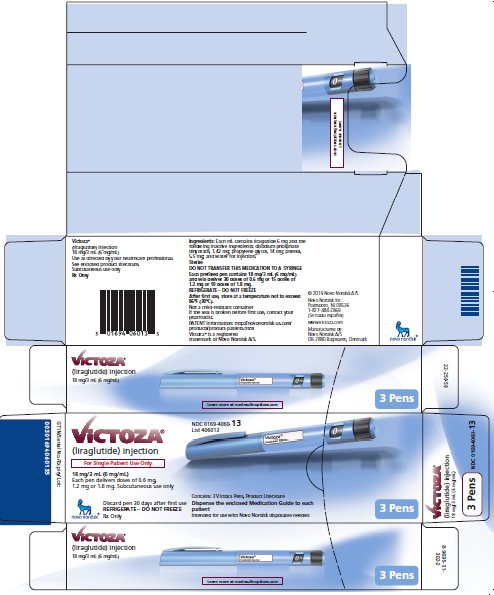
PRINCIPAL DISPLAY PANEL
NDC 0169-4060-13
List 406013
VICTOZA®
(liraglutide) injection
For Single Patient Use Only
18 mg/3 mL (6 mg/mL)
Each pen delivers doses of 0.6 mg, 1.2 mg or 1.8 mg
Subcutaneous use only
Discard pen 30 days after first use
REFRIGERATE - DO NOT FREEZE
Rx Only
Contains: 3 Victoza Pens, Product Literature
Dispense the enclosed Medication Guide to each patient
Intended for use with Novo Nordisk disposable needles
3 Pens
PRINCIPAL DISPLAY PANEL
NDC 0169-4060-12
List 406012
VICTOZA®
(liraglutide) injection
For Single Patient Use Only
18 mg/3 mL (6 mg/mL)
Each pen delivers doses of 0.6 mg, 1.2 mg or 1.8 mg
Subcutaneous use only
Discard pen 30 days after first use
REFRIGERATE - DO NOT FREEZE
Rx Only
Contains: 2 Victoza Pens, Product Literature
Dispense the enclosed Medication Guide to each patient
Intended for use with Novo Nordisk disposable needles
2 Pens