NDC Code(s) : 0093-3542-56, 0093-3543-56, 0093-3544-56, 0093-3545-56, 0093-3546-56, 0093-3547-56, 0093-3548-56
Packager : Teva Pharmaceuticals USA, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| AtomoxetineAtomoxetine CAPSULE | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| AtomoxetineAtomoxetine CAPSULE | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| AtomoxetineAtomoxetine CAPSULE | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| AtomoxetineAtomoxetine CAPSULE | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| AtomoxetineAtomoxetine CAPSULE | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| AtomoxetineAtomoxetine CAPSULE | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| AtomoxetineAtomoxetine CAPSULE | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| LABELER - Teva Pharmaceuticals USA, Inc.(001627975) |
PRINCIPAL DISPLAY PANEL
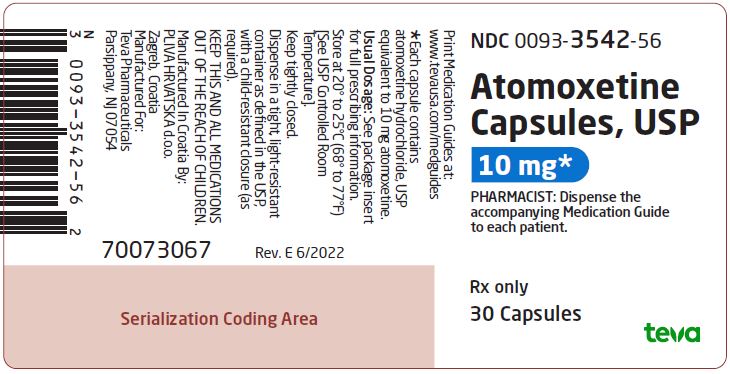
NDC 0093-3542-56
Atomoxetine Capsules, USP
10 mg*
PHARMACIST: Dispense the accompanying Medication Guide to each patient.
Rx only
30 Capsules
PRINCIPAL DISPLAY PANEL
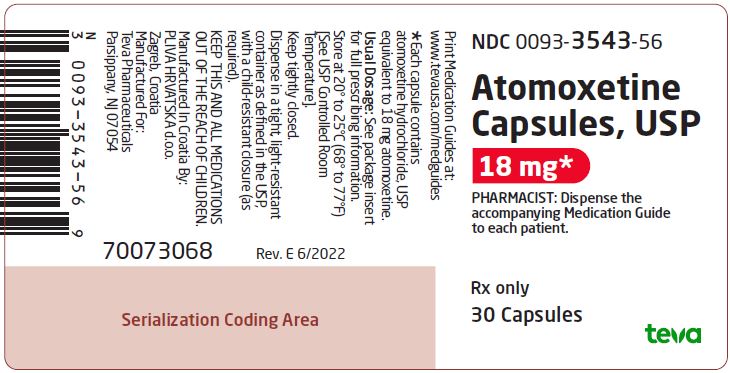
NDC 0093-3543-56
Atomoxetine Capsules, USP
18 mg*
PHARMACIST: Dispense the accompanying Medication Guide to each patient.
Rx only
30 Capsules
PRINCIPAL DISPLAY PANEL
NDC 0093-3544-56
Atomoxetine Capsules, USP
25 mg*
PHARMACIST: Dispense the accompanying Medication Guide to each patient.
Rx only
30 Capsules

PRINCIPAL DISPLAY PANEL
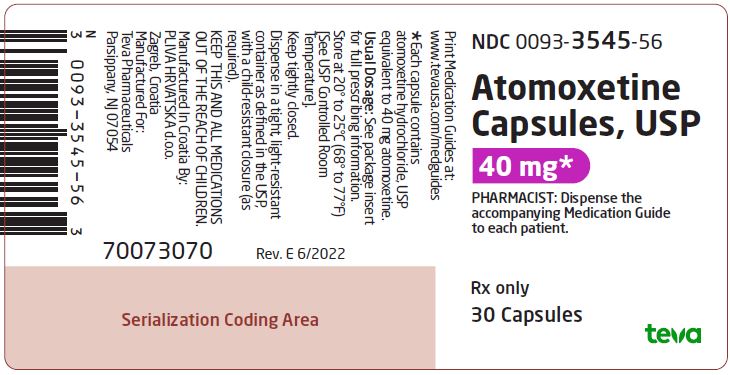
NDC 0093-3545-56
Atomoxetine Capsules, USP
40 mg*
PHARMACIST: Dispense the accompanying Medication Guide to each patient.
Rx only
30 Capsules
PRINCIPAL DISPLAY PANEL
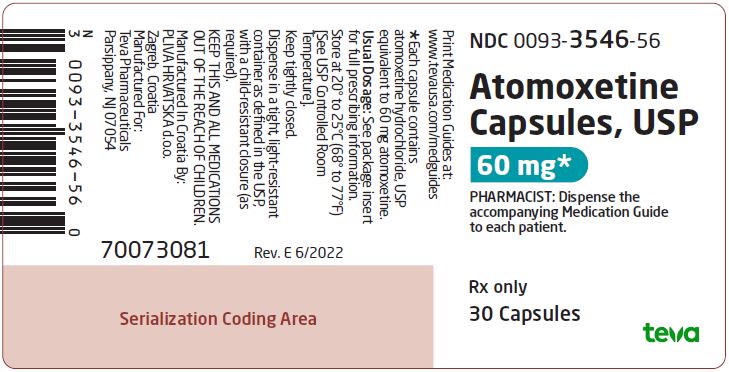
NDC 0093-3546-56
Atomoxetine Capsules, USP
60 mg*
PHARMACIST: Dispense the accompanying Medication Guide to each patient.
Rx only
30 Capsules
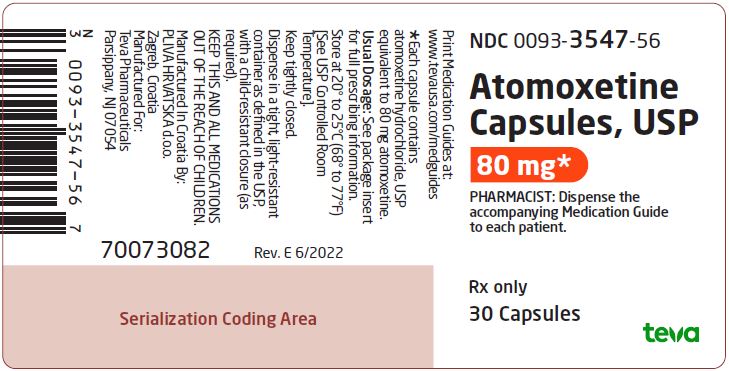
PRINCIPAL DISPLAY PANEL
NDC 0093-3547-56
Atomoxetine Capsules, USP
80 mg*
PHARMACIST: Dispense the accompanying Medication Guide to each patient.
Rx only
30 Capsules
PRINCIPAL DISPLAY PANEL
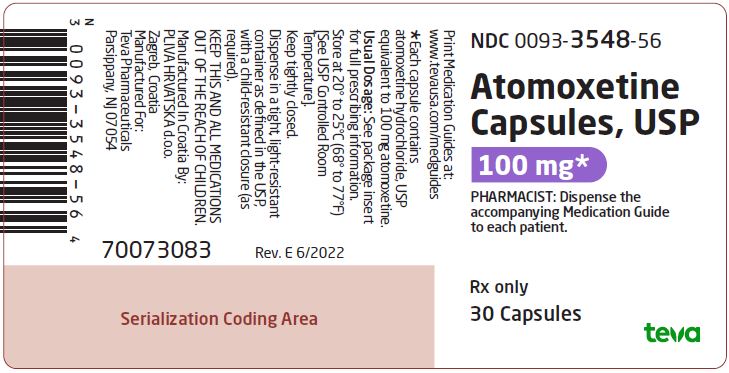
NDC 0093-3548-56
Atomoxetine Capsules, USP
100 mg*
PHARMACIST: Dispense the accompanying Medication Guide to each patient.
Rx only
30 Capsules