NDC Code(s) : 0032-1203-70, 0032-1203-64, 0032-1206-07, 0032-1206-01, 0032-1206-56, 0032-1212-07, 0032-1212-01, 0032-1212-46, 0032-1212-13, 0032-1224-07, 0032-1224-01, 0032-1224-46, 0032-3016-28, 0032-3016-13, 0032-3016-50, 0032-3016-12, 0032-0045-70, 0032-0045-30, 0032-0046-30, 0032-0046-70, 0032-0047-30, 0032-0047-70, 0032-2636-70, 0032-2636-01, 0032-2636-30, 0032-2637-70, 0032-2637-01, 0032-2637-30
Packager : AbbVie Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CreonPancrelipase CAPSULE, DELAYED RELEASE | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| CreonPancrelipase CAPSULE, DELAYED RELEASE PELLETS | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| CreonPancrelipase CAPSULE, DELAYED RELEASE PELLETS | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| CreonPancrelipase CAPSULE, DELAYED RELEASE PELLETS | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| CreonPancrelipase CAPSULE, DELAYED RELEASE PELLETS | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| CreonPancrelipase CAPSULE, DELAYED RELEASE | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| CreonPancrelipase CAPSULE, DELAYED RELEASE PELLETS | ||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| CreonPancrelipase CAPSULE, DELAYED RELEASE PELLETS | ||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| CreonPancrelipase CAPSULE, DELAYED RELEASE PELLETS | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| CreonPancrelipase CAPSULE, DELAYED RELEASE PELLETS | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - AbbVie Inc.(078458370) |
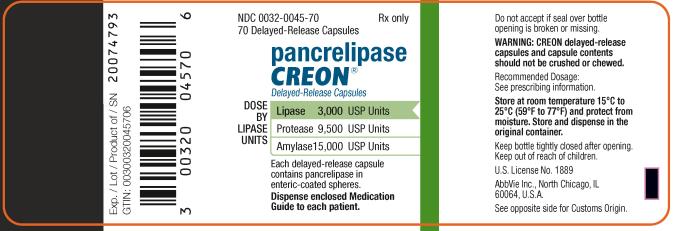
PRINCIPAL DISPLAY PANEL
NDC 0032-0045-70
70 Delayed-Release Capsules
Rx only
pancrelipase
CREON
®
Delayed-Release Capsules
DOSE BY LIPASE UNITS:
Lipase 3,000 USP Units
Protease 9,500 USP Units
Amylase 15,000 USP Units
Each delayed-release capsule contains
pancrelipase in enteric-coasted spheres.
Dispense enclosed Medication Guide to each patient.
PRINCIPAL DISPLAY PANEL
NDC 0032-0046-70
100 Delayed-Release Capsules
Rx only
pancrelipase
CREON
®
Delayed-Release Capsules
DOSE BY LIPASE UNITS:
Lipase 6,000 USP Units
Protease 19,000 USP Units
Amylase 30,000 USP Units
Each delayed-release capsule contains
pancrelipase in enteric-coasted spheres.
Dispense enclosed Medication Guide to each patient.
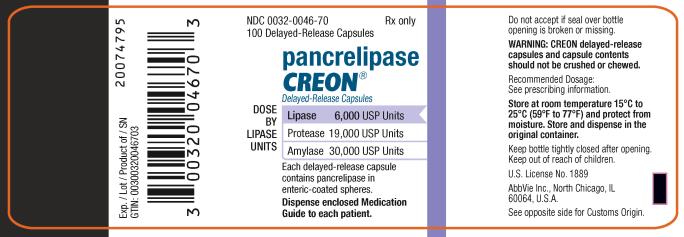
PRINCIPAL DISPLAY PANEL
NDC 0032-0047-70
100 Delayed-Release Capsules
Rx only
pancrelipase
CREON
®
Delayed-Release Capsules
DOSE BY LIPASE UNITS:
Lipase 12,000 USP Units
Protease 38,000 USP Units
Amylase 60,000 USP Units
Each delayed-release capsule contains
pancrelipase in enteric-coasted spheres.
Dispense enclosed Medication Guide to each patient.
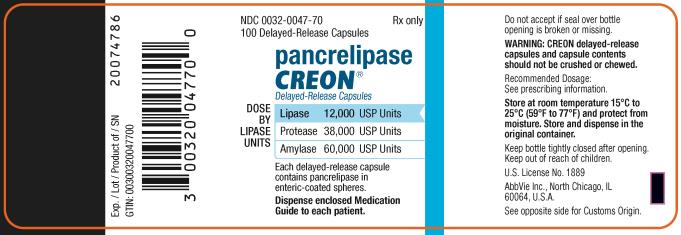
PRINCIPAL DISPLAY PANEL
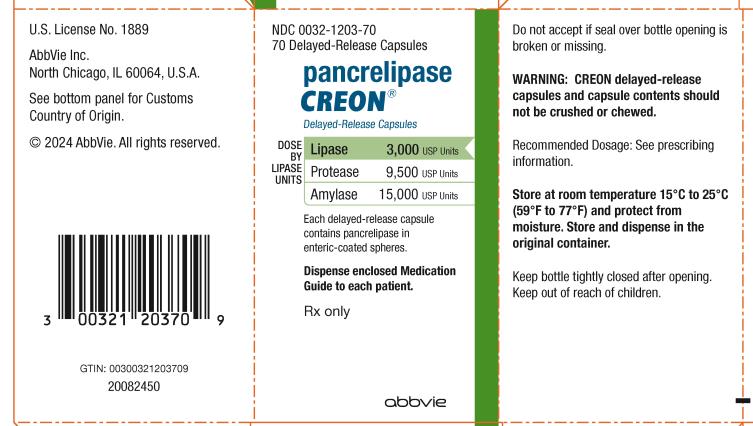
NDC 0032-1203-70
70 Delayed-Release Capsules
Pancrelipase
CREON
®
Delayed-Release Capsules
DOSE BY LIPASE UNITS:
Lipase 3,000 USP Units
Protease 9,500 USP Units
Amylase 15,000 USP Units
Each delayed-release capsule
contains pancrelipase in
enteric-coated spheres.
Dispense enclosed Medication
Guide to each patient.
Rx only
abbvie
PRINCIPAL DISPLAY PANEL
NDC 0032-1206-01
100 Delayed-Release Capsules
pancrelipase
CREON
®
Delayed-Release Capsules
DOSE BY LIPASE UNITS:
Lipase 6,000 USP Units
Protease 19,000 USP Units
Amylase 30,000 USP Units
Each delayed-release capsule contains
pancrelipase in enteric-coated spheres.
Dispense enclosed Medication
Guide to each patient.
Rx only
abbvie

PRINCIPAL DISPLAY PANEL
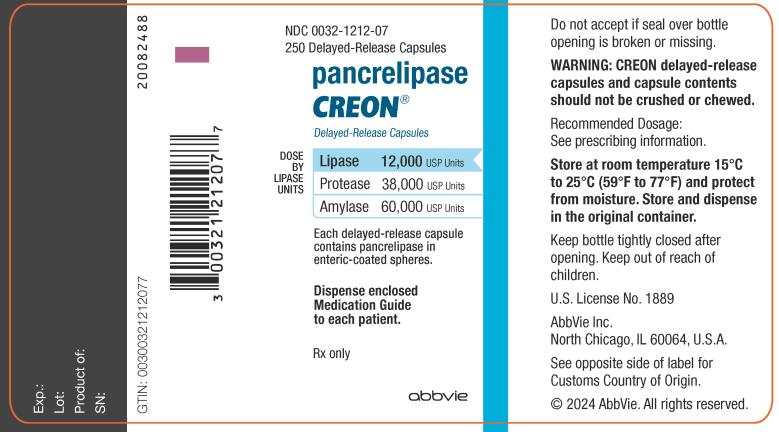
NDC 0032-1212-07
250 Delayed-Release Capsules
pancrelipase
CREON
®
Delayed-Release Capsules
DOSE BY LIPASE UNITS:
Lipase 12,000 USP Units
Protease 38,000 USP Units
Amylase 60,000 USP Units
Each delayed-release capsule
contains pancrelipase in
enteric-coated spheres.
Dispense enclosed
Medication Guide
to each patient.
Rx only
abbvie
PRINCIPAL DISPLAY PANEL
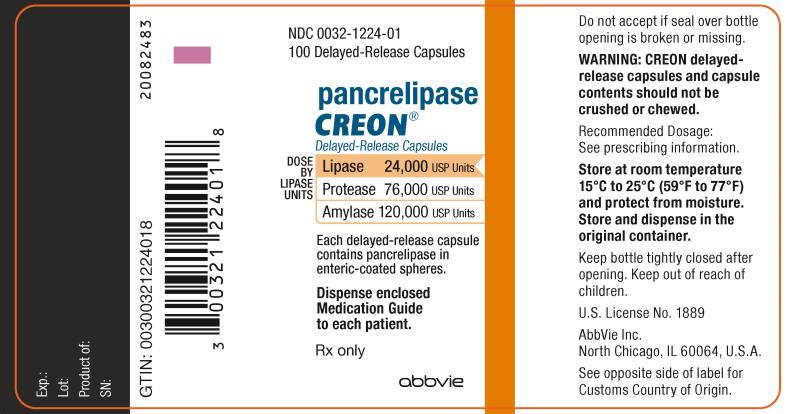
NDC 0032-1224-01
100 Delayed-Release Capsules
pancrelipase
CREON
®
Delayed-Release Capsules
DOSE BY LIPASE UNITS:
Lipase 24,000 USP Units
Protease 76,000 USP Units
Amylase 120,000 USP Units
Each delayed-release capsule
contains pancrelipase in
enteric-coated spheres.
Dispense enclosed
Medication Guide
to each patient.
Rx only
abbvie
PRINCIPAL DISPLAY PANEL
NDC 0032-2636-70
240 Delayed-Release Capsules
pancrelipase
CREON
®
Delayed-Release Capsules
DOSE BY LIPASE UNITS:
Lipase 24,000 USP Units
Protease 76,000 USP Units
Amylase 120,000 USP Unites
Each delayed-release capsule contains
pancrelipase in enteric-coasted spheres.
Dispense enclosed Medication Guide to each patient.
Rx only
Abbvie

PRINCIPAL DISPLAY PANEL
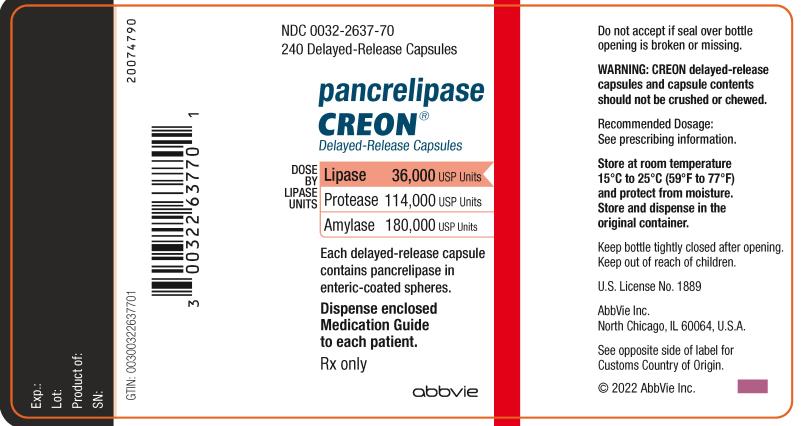
NDC 0032-2637-70
240 Delayed-Release Capsules
pancrelipase
CREON
®
Delayed-Release Capsules
DOSE BY LIPASE UNITS:
Lipase 36,000 USP Units
Protease 114,000 USP Units
Amylase 180,000 USP Unites
Each delayed-release capsule contains
pancrelipase in enteric-coasted spheres.
Dispense enclosed Medication
Guide to each patient.
Rx only
Abbvie
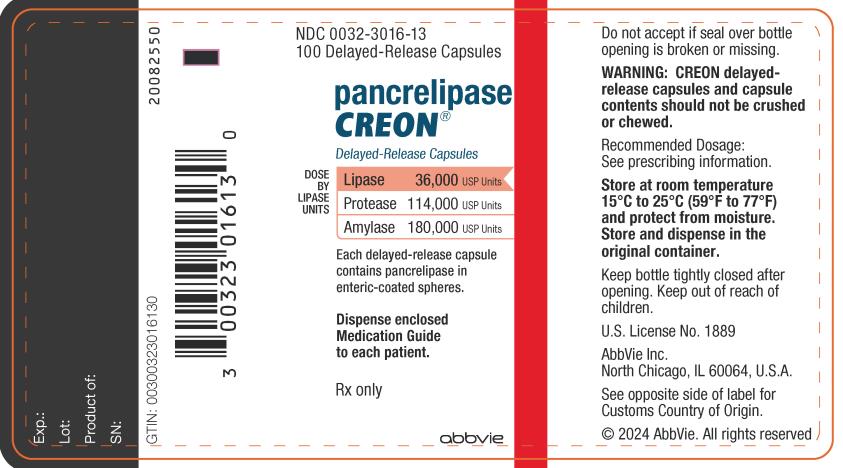
PRINCIPAL DISPLAY PANEL
NDC 0032-3016-13
100 Delayed-Release Capsules
pancrelipase
CREON
®
Delayed-Release Capsules
CREON® (pancrelipase)
Delayed-Release Capsules
DOSE BY LIPASE UNITS:
Lipase 36,000 USP Units
Protease 114,000 USP Units
Amylase 180,000 USP Units
Each delayed-release capsule
contains pancrelipase in
enteric-coated spheres.
Dispense enclosed
Medication Guide
to each patient.
Rx only
abbvie