NDC Code(s) : 0019-9452-75, 0019-9452-01, 0019-9452-02, 0019-9452-03, 0019-9452-04, 0019-9452-05, 0019-9452-06, 0019-9452-07, 0019-9452-08, 0019-9452-09, 0019-9452-10, 0019-9452-11, 0019-9452-12, 0019-9452-15, 0019-9452-17, 0019-9452-20, 0019-9452-50, 0019-9452-00
Packager : Mallinckrodt Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| SODIUM IODIDE I 131sodium iodide i 131 CAPSULE, GELATIN COATED | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
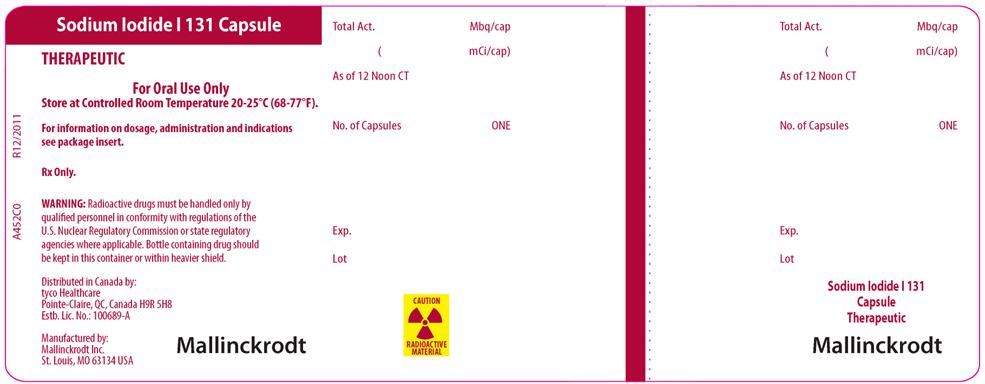
PRINCIPAL DISPLAY PANEL
SODIUM IODIDE I 131 CAPSULE
THERAPEUTIC
For Oral Use Only
Store at Controlled Room Temperature 20-25°C (68-77°F).
For information on dosage, administration and indications see package insert.
Rx Only.
WARNING: Radioactive drugs must be handled only by qualified personnel in conformity with regulations of the U.S. Nuclear Regulatory Commission or state regulatory agencies where applicable. Bottle containing drug should be kept in this container or within heavier shield.
Distributed in Canada:
tyco Healthcare
Pointe-Claire, QC, Canada H9R 5H8
Est. Lic. No 100689-A
Manufactured by:
Mallinckrodt Inc.
St. Louis, MO 63134 USA
MALLINCKRODT
CAUTION RADIOACTIVE MATERIAL
A452C0
R12/2011