NDC Code(s) : 0002-8805-01, 0002-8805-59, 0002-8805-99
Packager : Eli Lilly and Company
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Humulin Insulin human INJECTION, SUSPENSION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
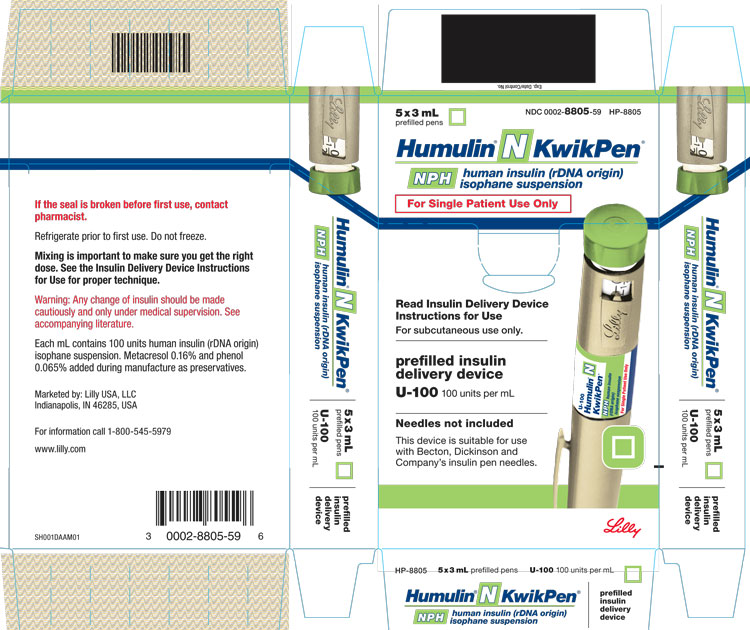
PRINCIPAL DISPLAY PANEL
5x3 mL
prefilled pens
NDC 0002-8805-59
HP-8805
Humulin® N KwikPen®
NPH
human insulin (rDNA origin)
isophane suspension
For Single Patient Use Only
Read Insulin Delivery Device Instructions for Use
For subcutaneous use only.
prefilled insulin delivery device
U-100 100 units per mL
Needles not included
This device is suitable for use with Becton, Dickinson and Company's insulin pen needles.
Lilly