Vitamin B12 marketed by Hikma Pharmaceuticals USA Inc. under NDC Code 0143-9619-01
NDC Code(s) : 0143-9621-01, 0143-9621-25, 0143-9620-01, 0143-9620-10, 0143-9619-01, 0143-9619-10
Packager : Hikma Pharmaceuticals USA Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Cyanocobalamincyanocobalamin INJECTION |
|
|
|
|
|
|
|
|
|
|
| Cyanocobalamincyanocobalamin INJECTION |
|
|
|
|
|
|
|
|
|
|
| Cyanocobalamincyanocobalamin INJECTION |
|
|
|
|
|
|
|
|
|
|
PRINCIPAL DISPLAY PANEL
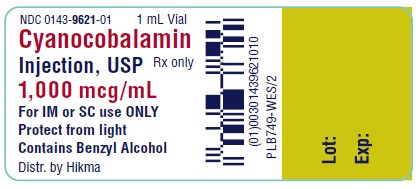
NDC 0143-9621-01 1 mL Vial
Cyanocobalamin
Injection, USP
Rx only
1,000 mcg/mL
For IM or SC use ONLY
Protect from light
Contains Benzyl Alcohol
NDC 0143-9621-25 Rx only
Cyanocobalamin
Injection, USP
1,000 mcg/mL
For Intramuscular or
Subcutaneous use ONLY
Contains Benzyl Alcohol
as a Preservative
25 x 1 mL Vials

PRINCIPAL DISPLAY PANEL
NDC 0143-9620-01 10 mL Multiple Dose Vial
Cyanocobalamin
Injection, USP
Rx only
10,000 mcg per 10 mL
(1,000 mcg/mL)
For Intramuscular or
Subcutaneous use ONLY
Contains Benzyl Alcohol
as a Preservative

NDC 0143-9620-10 Rx only
Cyanocobalamin
Injection, USP
10,000 mcg per 10 mL
(1,000 mcg/mL)
For Intramuscular or
Subcutaneous use ONLY
Contains Benzyl Alcohol
as a Preservative
10 x 10 mL Multiple Dose Vials

PRINCIPAL DISPLAY PANEL
NDC 0143-9619-01 30 mL Multiple Dose Vial
Cyanocobalamin
Injection, USP
Rx only
30,000 mcg per 30 mL
(1,000 mcg/mL)
For Intramuscular or
Subcutaneous use ONLY
Contains Benzyl Alcohol
as a Preservative

NDC 0143-9619-10 Rx only
Cyanocobalamin
Injection, USP
30,000 mcg per 30 mL
(1,000 mcg/mL)
For Intramuscular or
Subcutaneous use ONLY
Contains Benzyl Alcohol
as a Preservative
10 x 30 mL Multiple Dose Vials

NDC 0143-9619-01 Rx only
Cyanocobalamin
Injection, USP
30,000 mcg per 30 mL
(1,000 mcg/mL)
For Intramuscular or
Subcutaneous use ONLY
Contains Benzyl Alcohol
as a Preservative
1 x 30 mL Multiple Dose Vials

Copyright © 2024 LePro PharmaCompass OPC Private Limited, All rights reserved.














