

Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
FDF
0
Europe

0
Australia

0
South Africa

0
EDQM
0
USP
0
JP
0
Others
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
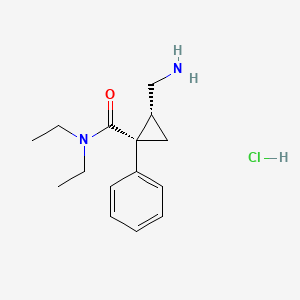

1. Fetzima
2. Levomilnacipran
1. 175131-60-9
2. Milnacipran Hydrochloride
3. (1s-cis)-milnacipran Hydrochloride
4. Fetzima
5. F 2695 Hydrochloride
6. 101152-94-7
7. Milnacipran ((1s-cis) Hydrochloride)
8. Milnacipran (1s-cis) Hydrochloride
9. Levomilnacipran Hcl
10. F2695 Hydrochloride
11. Levomilnacipran Hydrochloride [usan]
12. F-2695 Hydrochloride
13. 371u2zk31u
14. (1s,2r)-2-(aminomethyl)-n,n-diethyl-1-phenylcyclopropane-1-carboxamide;hydrochloride
15. (1s-cis)-milnacipran Hcl
16. (1s,2r)-2-(aminomethyl)-n,n-diethyl-1-phenylcyclopropanecarboxamide Hydrochloride
17. Levomilnacipran Hydrochloride (usan)
18. Cyclopropanecarboxamide, 2-(aminomethyl)-n,n-diethyl-1-phenyl-, Hydrochloride (1:1), (1s,2r)-
19. Midalcipran Hydrochloride
20. Rac-milnacipran Hydrochloride
21. Joncia
22. Unii-371u2zk31u
23. L-milnacipran Hcl
24. (1s,2r)-milnacipran Hydrochloride
25. Fetzima (tn)
26. Tn-912
27. Schembl1148742
28. Chembl2105732
29. Hy-b0168b
30. Milnacipran Hydrochloride Solution
31. Dtxsid801026201
32. Amy10333
33. Mfcd18433402
34. S5693
35. Akos025311514
36. Ccg-231710
37. Cs-4972
38. (1s,2r)-2-(aminomethyl)-n,n-diethyl-1-phenylcyclopropane-1-carboxamide Hydrochloride
39. As-12868
40. M2133
41. Milnacipran Hydrochloride, (1s,2r)-
42. Levomilnacipran Hydrochloride [who-dd]
43. A14391
44. C13985
45. C73344
46. D10133
47. F-2207
48. 152m947
49. A899431
50. Levomilnacipran Hydrochloride [orange Book]
51. Q27256611
52. Milnacipran Hydrochloride 1.0 Mg/ml In Methanol (as Free Base)
| Molecular Weight | 282.81 g/mol |
|---|---|
| Molecular Formula | C15H23ClN2O |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 5 |
| Exact Mass | 282.1498911 g/mol |
| Monoisotopic Mass | 282.1498911 g/mol |
| Topological Polar Surface Area | 46.3 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 295 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Fetzima |
| PubMed Health | Levomilnacipran (By mouth) |
| Drug Classes | Antidepressant |
| Active Ingredient | Levomilnacipran hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | eq 40mg base; eq 80mg base; eq 120mg base; eq 20mg base |
| Market Status | Prescription |
| Company | Forest Labs |
| 2 of 2 | |
|---|---|
| Drug Name | Fetzima |
| PubMed Health | Levomilnacipran (By mouth) |
| Drug Classes | Antidepressant |
| Active Ingredient | Levomilnacipran hydrochloride |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | eq 40mg base; eq 80mg base; eq 120mg base; eq 20mg base |
| Market Status | Prescription |
| Company | Forest Labs |
Antidepressive Agents
Mood-stimulating drugs used primarily in the treatment of affective disorders and related conditions. Several MONOAMINE OXIDASE INHIBITORS are useful as antidepressants apparently as a long-term consequence of their modulation of catecholamine levels. The tricyclic compounds useful as antidepressive agents (ANTIDEPRESSIVE AGENTS, TRICYCLIC) also appear to act through brain catecholamine systems. A third group (ANTIDEPRESSIVE AGENTS, SECOND-GENERATION) is a diverse group of drugs including some that act specifically on serotonergic systems. (See all compounds classified as Antidepressive Agents.)
Serotonin and Noradrenaline Reuptake Inhibitors
Drugs that selectively block or suppress the plasma membrane transport of SEROTONIN and NORADRENALINE into axon terminals and are used as ANTIDEPRESSIVE AGENTS. (See all compounds classified as Serotonin and Noradrenaline Reuptake Inhibitors.)
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
