

Synopsis
Synopsis
0
EU WC
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
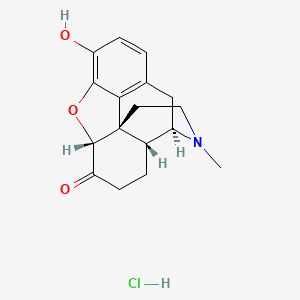

1. Dihydromorphinone
2. Dilaudid
3. Hydromorphon
4. Hydromorphone
5. Laudacon
6. Palladone
1. 71-68-1
2. Hydromorphone Hcl
3. Dilaudid
4. Palladone
5. Hymorphan
6. Exalgo
7. Dihydromorphinone Hydrochloride
8. Dilaudid-hp
9. L960up2krw
10. Hydromorphone Hydrochloride Cii
11. Nsc-117862
12. Dea No. 9150
13. (4r,4ar,7ar,12bs)-9-hydroxy-3-methyl-1,2,4,4a,5,6,7a,13-octahydro-4,12-methanobenzofuro[3,2-e]isoquinolin-7-one;hydrochloride
14. 4,5-alpha-epoxy-3-hydroxy-17-methylmorphinan-6-one Hydrochloride
15. Chebi:5791
16. Morphinone, Dihydro-, Hydrochloride
17. Einecs 200-762-6
18. Unii-l960up2krw
19. Nsc 117862
20. Hydromorphone Hydrochloride [usp]
21. Palladone (tn)
22. Dilaudid (tn)
23. (4r,4ar,7ar,12bs)-9-hydroxy-3-methyl-1,2,4,4a,5,6,7a,13-octahydro-4,12-methanobenzofuro[3,2-e]isoquinoline-7-one;hydrochloride
24. Exalgo (tn)
25. 4,5alpha-epoxy-3-hydroxy-17-methylmorphinan-6-one Hydrochloride
26. Schembl30521
27. Hydromorphone Hydrochloride Usp
28. Chembl1237055
29. Dtxsid90991291
30. Morphinan-6-one, 4,5alpha-epoxy-3-hydroxy-17-methyl-, Hydrochloride
31. Hydromorphone Hydrochloride (jan/usp)
32. Morphinan-6-one, 4,5-epoxy-3-hydroxy-17-methyl-, Hydrochloride, (5alpha)-
33. Hydromorphone Hydrochloride [mi]
34. Morphinan-6-one, 4,5-alpha-epoxy-3-hydroxy-17-methyl-, Hydrochloride, (5'-alpha)-
35. Hydromorphone Hydrochloride [jan]
36. Hydromorphone Hydrochloride [mart.]
37. Hydromorphone Hydrochloride [vandf]
38. Hydromorphone Hydrochloride [who-dd]
39. D00839
40. Hydromorphone Hydrochloride [orange Book]
41. Hydromorphone Hydrochloride Cii [usp-rs]
42. Hydromorphone Hydrochloride [ep Monograph]
43. Hydromorphone Hydrochloride [usp Monograph]
44. Q27106892
45. 4,5.alpha.-epoxy-3-hydroxy-17-methylmorphinan-6-one Hydrochloride
46. Hydromorphone Hydrochloride Solution, Drug Standard, 1.0 Mg/ml In Methanol
47. Hydromorphone Hydrochloride, European Pharmacopoeia (ep) Reference Standard
48. Hydromorphone Hydrochloride, United States Pharmacopeia (usp) Reference Standard
49. Morphinan-6-one, 4,5-epoxy-3-hydroxy-17-methyl-, Hydrochloride (1:1), (5.alpha.)
50. Morphinan-6-one, 4,5-epoxy-3-hydroxy-17-methyl-, Hydrochloride, (5.alpha.)-
| Molecular Weight | 321.8 g/mol |
|---|---|
| Molecular Formula | C17H20ClNO3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 321.1131712 g/mol |
| Monoisotopic Mass | 321.1131712 g/mol |
| Topological Polar Surface Area | 49.8 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 494 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Exalgo |
| Drug Label | EXALGO tablets contain hydromorphone hydrochloride, a mu-opioid agonist.Hydromorphone hydrochloride USP is 4,5-epoxy-3-hydroxy-17-methlymorphinan-6-one hydrochloride. Hydromorphone hydrochloride is a white or almost white crystalline powder that... |
| Active Ingredient | Hydromorphone hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 32mg; 12mg; 16mg; 8mg |
| Market Status | Prescription |
| Company | Mallinckrodt |
| 2 of 4 | |
|---|---|
| Drug Name | Hydromorphone hydrochloride |
| PubMed Health | Hydromorphone |
| Drug Classes | Analgesic, Anesthetic Adjunct, Central Nervous System Agent |
| Drug Label | Hydromorphone Hydrochloride Tablets USP, 2 mg, 4 mg, and 8 mg are supplied in tablet form for oral administration.Hydromorphone hydrochloride, a hydrogenated ketone of morphine, is an opioid analgesic.The chemical name of hydromorphone hydrochloride... |
| Active Ingredient | Hydromorphone hydrochloride |
| Dosage Form | Tablet, extended release; Tablet; Injectable; Solution |
| Route | Injection; Oral |
| Strength | 2mg/ml; 1mg/ml; 8mg; 10mg/ml; 4mg; 4mg/ml; 12mg; 2mg; 16mg; 5mg/5ml |
| Market Status | Prescription |
| Company | Hospira; Actavis Labs Fl; Mallinckrodt; Roxane; Lannett; Elite Labs; Akorn; Barr |
| 3 of 4 | |
|---|---|
| Drug Name | Exalgo |
| Drug Label | EXALGO tablets contain hydromorphone hydrochloride, a mu-opioid agonist.Hydromorphone hydrochloride USP is 4,5-epoxy-3-hydroxy-17-methlymorphinan-6-one hydrochloride. Hydromorphone hydrochloride is a white or almost white crystalline powder that... |
| Active Ingredient | Hydromorphone hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 32mg; 12mg; 16mg; 8mg |
| Market Status | Prescription |
| Company | Mallinckrodt |
| 4 of 4 | |
|---|---|
| Drug Name | Hydromorphone hydrochloride |
| PubMed Health | Hydromorphone |
| Drug Classes | Analgesic, Anesthetic Adjunct, Central Nervous System Agent |
| Drug Label | Hydromorphone Hydrochloride Tablets USP, 2 mg, 4 mg, and 8 mg are supplied in tablet form for oral administration.Hydromorphone hydrochloride, a hydrogenated ketone of morphine, is an opioid analgesic.The chemical name of hydromorphone hydrochloride... |
| Active Ingredient | Hydromorphone hydrochloride |
| Dosage Form | Tablet, extended release; Tablet; Injectable; Solution |
| Route | Injection; Oral |
| Strength | 2mg/ml; 1mg/ml; 8mg; 10mg/ml; 4mg; 4mg/ml; 12mg; 2mg; 16mg; 5mg/5ml |
| Market Status | Prescription |
| Company | Hospira; Actavis Labs Fl; Mallinckrodt; Roxane; Lannett; Elite Labs; Akorn; Barr |
Analgesics, Opioid
Compounds with activity like OPIATE ALKALOIDS, acting at OPIOID RECEPTORS. Properties include induction of ANALGESIA or NARCOSIS. (See all compounds classified as Analgesics, Opioid.)
Narcotics
Agents that induce NARCOSIS. Narcotics include agents that cause somnolence or induced sleep (STUPOR); natural or synthetic derivatives of OPIUM or MORPHINE or any substance that has such effects. They are potent inducers of ANALGESIA and OPIOID-RELATED DISORDERS. (See all compounds classified as Narcotics.)
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
