
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
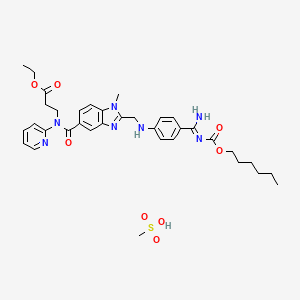

1. Bibr 1048
2. Dabigatran
3. Dabigatran Etexilate
4. Dabigatran Etexilate Mesylate
5. Etexilate Mesylate, Dabigatran
6. Etexilate, Dabigatran
7. Mesylate, Dabigatran Etexilate
8. N-((2-(((4-(aminoiminomethyl)phenyl)amino)methyl)-1-methyl-1h-benzimidazol-5-yl)carbonyl)-n-2-pyridinyl-beta-alanine
9. Pradaxa
1. Dabigatran Etexilate Mesylate
2. 872728-81-9
3. Pradaxa
4. Ethyl 3-[[2-[[4-[(z)-n'-hexoxycarbonylcarbamimidoyl]anilino]methyl]-1-methylbenzimidazole-5-carbonyl]-pyridin-2-ylamino]propanoate;methanesulfonic Acid
5. Dtxsid20236248
6. Ex-a1966
7. Mfcd25424070
8. S5960
9. Ccg-213236
10. Cs-w004358
11. (z)-ethyl 3-(2-(((4-(n'-((hexyloxy)carbonyl)carbamimidoyl)phenyl)amino)methyl)-1-methyl-n-(pyridin-2-yl)-1h-benzo[d]imidazole-5-carboxamido)propanoate Methanesulfonate
12. 728p819
| Molecular Weight | 723.8 g/mol |
|---|---|
| Molecular Formula | C35H45N7O8S |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 17 |
| Exact Mass | 723.30503259 g/mol |
| Monoisotopic Mass | 723.30503259 g/mol |
| Topological Polar Surface Area | 217 Ų |
| Heavy Atom Count | 51 |
| Formal Charge | 0 |
| Complexity | 1100 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Pradaxa |
| PubMed Health | Dabigatran (By mouth) |
| Drug Classes | Anticoagulant |
| Drug Label | The chemical name for dabigatran etexilate mesylate, a direct thrombin inhibitor, is -Alanine, N-[[2-[[[4-[[[(hexyloxy)carbonyl]amino]iminomethyl] phenyl]amino]methyl]-1-methyl-1H-benzimidazol-5-yl]carbonyl]-N-2-pyridinyl-,ethyl ester, methanesulfo... |
| Active Ingredient | Dabigatran etexilate mesylate |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 150mg base; eq 75mg base |
| Market Status | Prescription |
| Company | Boehringer Ingelheim |
| 2 of 2 | |
|---|---|
| Drug Name | Pradaxa |
| PubMed Health | Dabigatran (By mouth) |
| Drug Classes | Anticoagulant |
| Drug Label | The chemical name for dabigatran etexilate mesylate, a direct thrombin inhibitor, is -Alanine, N-[[2-[[[4-[[[(hexyloxy)carbonyl]amino]iminomethyl] phenyl]amino]methyl]-1-methyl-1H-benzimidazol-5-yl]carbonyl]-N-2-pyridinyl-,ethyl ester, methanesulfo... |
| Active Ingredient | Dabigatran etexilate mesylate |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 150mg base; eq 75mg base |
| Market Status | Prescription |
| Company | Boehringer Ingelheim |
Pradaxa 75 mg
- Primary prevention of venous thromboembolic events in adult patients who have undergone elective total hip replacement surgery or total knee replacement surgery.
Pradaxa 110 mg
- Primary prevention of venous thromboembolic events in adult patients who have undergone elective total hip replacement surgery or total knee replacement surgery.
- Prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation (NVAF), with one or more risk factors, such as prior stroke or transient ischemic attack (TIA); age 75 years; heart failure (NYHA Class II); diabetes mellitus; hypertension.
- Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults.
Pradaxa 150 mg
- Prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation (NVAF), with one or more risk factors, such as prior stroke or transient ischemic attack (TIA); age 75 years; heart failure (NYHA Class II); diabetes mellitus; hypertension.
- Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults.
Antithrombins
Endogenous factors and drugs that directly inhibit the action of THROMBIN, usually by blocking its enzymatic activity. They are distinguished from INDIRECT THROMBIN INHIBITORS, such as HEPARIN, which act by enhancing the inhibitory effects of antithrombins. (See all compounds classified as Antithrombins.)
B01AE07

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Reply
18 Oct 2024
Reply
08 Oct 2024
Reply
04 Sep 2024
Reply
13 Feb 2023
Reply
06 Sep 2022
Reply
06 Oct 2021
Reply
19 May 2021
Reply
12 Mar 2021
Reply
29 Jan 2021
Reply
15 Oct 2020
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Reply
08 Oct 2022
Reply
22 Jun 2022
Reply
31 Jan 2022
Reply
19 Jun 2021
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Patents & EXCLUSIVITIES
Patent Expiration Date : 2031-01-20
US Patent Number : 9034822
Drug Substance Claim :
Drug Product Claim :
Application Number : 22512
Patent Use Code : U-1759
Delist Requested :
Patent Use Description : METHOD OF REVERSING TH...
Patent Expiration Date : 2031-01-20

Patent Expiration Date : 2026-03-07
US Patent Number : 7932273*PED
Drug Substance Claim :
Drug Product Claim :
Application Number : 22512
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2026-03-07

Patent Expiration Date : 2026-03-07
US Patent Number : 7932273*PED
Drug Substance Claim :
Drug Product Claim :
Application Number : 214358
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2026-03-07

Patent Expiration Date : 2031-01-20
US Patent Number : 9034822
Drug Substance Claim :
Drug Product Claim :
Application Number : 22512
Patent Use Code : U-1759
Delist Requested :
Patent Use Description : METHOD OF REVERSING TH...
Patent Expiration Date : 2031-01-20

Patent Expiration Date : 2026-03-07
US Patent Number : 7932273*PED
Drug Substance Claim :
Drug Product Claim :
Application Number : 214358
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2026-03-07

Patent Expiration Date : 2026-03-07
US Patent Number : 7932273*PED
Drug Substance Claim :
Drug Product Claim :
Application Number : 22512
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2026-03-07

Patent Expiration Date : 2031-01-20
US Patent Number : 9034822
Drug Substance Claim :
Drug Product Claim :
Application Number : 22512
Patent Use Code : U-1759
Delist Requested :
Patent Use Description : METHOD OF REVERSING TH...
Patent Expiration Date : 2031-01-20

Patent Expiration Date : 2026-03-07
US Patent Number : 7932273*PED
Drug Substance Claim :
Drug Product Claim :
Application Number : 214358
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2026-03-07

Patent Expiration Date : 2031-07-20
US Patent Number : 9034822*PED
Drug Substance Claim :
Drug Product Claim :
Application Number : 22512
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2031-07-20

Patent Expiration Date : 2025-09-07
US Patent Number : 7932273
Drug Substance Claim : Y
Drug Product Claim : Y
Application Number : 214358
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2025-09-07

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?