

Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Acid, Azolinic
2. Azolinic Acid
3. Cinobac
4. Clinoxacin
5. Compound 64716
1. 28657-80-9
2. Cinobac
3. Compound 64716
4. Cinoxacino
5. Cinoxacinum
6. Cinobactin
7. Cinoxacine
8. Uronorm
9. Cinx
10. 1-ethyl-6,7-methylenedioxy-4(1h)-oxocinnoline-3-carboxylic Acid
11. Nsc 304467
12. Mls000069630
13. 1-ethyl-4-oxo-[1,3]dioxolo[4,5-g]cinnoline-3-carboxylic Acid
14. 1-ethyl-1,4-dihydro-4-oxo(1,3)dioxolo(4,5-g)cinnoline-3-carboxylic Acid
15. Nsc-304467
16. Lmk22vuh23
17. 1-ethyl-1,4-dihydro-4-oxo[1,3]dioxolo[4,5-g]cinnoline-3-carboxylic Acid
18. Smr000058232
19. [1,3]dioxolo[4,5-g]cinnoline-3-carboxylic Acid, 1-ethyl-1,4-dihydro-4-oxo-
20. Chebi:3716
21. 1-ethyl-4-oxo-1,4-dihydro-[1,3]dioxolo[4,5-g]cinnoline-3-carboxylic Acid
22. 1-ethyl-4-oxo-1,4-dihydro[1,3]dioxolo[4,5-g]cinnoline-3-carboxylic Acid
23. Nsc304467
24. Ncgc00015277-02
25. Cas-28657-80-9
26. Dsstox_cid_2822
27. (1,3)dioxolo(4,5-g)cinnoline-3-carboxylic Acid, 1,4-dihydro-1-ethyl-4-oxo-
28. (1,3)dioxolo(4,5-g)cinnoline-3-carboxylic Acid, 1-ethyl-1,4-dihydro-4-oxo-
29. Dsstox_rid_76745
30. Cinoxacine [inn-french]
31. Cinoxacinum [inn-latin]
32. Dsstox_gsid_22822
33. Cinoxacino [inn-spanish]
34. Lilly 64716
35. 1-ethyl-4-oxo-1h,4h,7h-[1,3]dioxolo[4,5-g]cinnoline-3-carboxylic Acid
36. Cinobac (tn)
37. Sr-01000000129
38. Einecs 249-133-8
39. Unii-lmk22vuh23
40. Brn 1084304
41. Noxigram
42. Ccris 8206
43. Tnp00246
44. Prestwick_239
45. Cinoxacin [usan:usp:inn:ban:jan]
46. Spectrum_000152
47. Cinoxacin [inn]
48. Cinoxacin [jan]
49. Cinoxacin [mi]
50. Cinoxacin [usan]
51. Opera_id_1392
52. Prestwick0_000780
53. Prestwick1_000780
54. Prestwick2_000780
55. Prestwick3_000780
56. Spectrum2_000570
57. Spectrum3_000352
58. Spectrum4_000289
59. Spectrum5_000749
60. Cinoxacin [vandf]
61. Lopac-c-8645
62. Cinoxacin [mart.]
63. C 8645
64. Cid_2762
65. Cinoxacin [who-dd]
66. Chembl1208
67. Lopac0_000309
68. Oprea1_131085
69. Schembl43770
70. Bspbio_000860
71. Bspbio_002043
72. Kbiogr_000818
73. Kbioss_000632
74. Mls001148076
75. Divk1c_000318
76. Spectrum1500190
77. Spbio_000360
78. Spbio_002799
79. Cinoxacin (jp17/usp/inn)
80. Bpbio1_000946
81. Cinoxacin [orange Book]
82. Cinoxacin [usp Impurity]
83. Dtxsid8022822
84. Bdbm39350
85. Hms500p20
86. Kbio1_000318
87. Kbio2_000632
88. Kbio2_003200
89. Kbio2_005768
90. Kbio3_001263
91. Zinc32350
92. Ninds_000318
93. Hms1570k22
94. Hms1920o11
95. Hms2091e18
96. Hms2097k22
97. Hms2235k07
98. Hms3260n20
99. Hms3370b19
100. Hms3714k22
101. Pharmakon1600-01500190
102. Hy-b1085
103. Tox21_110121
104. Tox21_500309
105. Ccg-39160
106. Nsc756695
107. 1-ethyl-4-oxohydro-7h-1,3-dioxoleno[4,5-g]cinnoline-3-carboxylic Acid
108. Akos022507372
109. Tox21_110121_1
110. Cs-4652
111. Db00827
112. Lp00309
113. Nsc-756695
114. Sdccgsbi-0050297.p005
115. Idi1_000318
116. Cinoxacin 100 Microg/ml In Acetonitrile
117. Ncgc00015277-01
118. Ncgc00015277-03
119. Ncgc00015277-04
120. Ncgc00015277-05
121. Ncgc00015277-06
122. Ncgc00015277-07
123. Ncgc00015277-08
124. Ncgc00015277-09
125. Ncgc00015277-10
126. Ncgc00015277-11
127. Ncgc00015277-12
128. Ncgc00015277-13
129. Ncgc00015277-14
130. Ncgc00015277-17
131. Ncgc00015277-21
132. Ncgc00023754-03
133. Ncgc00023754-04
134. Ncgc00023754-05
135. Ncgc00023754-06
136. Ncgc00023754-07
137. Ncgc00260994-01
138. As-73151
139. Sbi-0050297.p004
140. Db-047427
141. Eu-0100309
142. Ft-0602947
143. Sw196546-3
144. C08052
145. D00872
146. Ab00051948_17
147. Ab00051948_18
148. A819523
149. J-017182
150. Q1639588
151. Sr-01000000129-2
152. Sr-01000000129-4
153. Sr-01000000129-7
154. Brd-k14704277-001-05-1
155. Brd-k14704277-001-15-0
156. 1-ethyl-1,3]dioxolo[4,5-g]cinnoline-3-carboxylic Acid
157. Cinoxacin, United States Pharmacopeia (usp) Reference Standard
158. [1,5-g]cinnoline-3-carboxylic Acid, 1-ethyl-1,4-dihydro-4-oxo-
159. 1-ethyl-1,4-dihydro-4-oxo(1,3)dioxolo(4,5-g)cinnoline-3-carboxylicacid
160. 1-ethyl-4-oxidanylidene-[1,3]dioxolo[4,5-g]cinnoline-3-carboxylic Acid
161. (1,3)dioxolo(4,5-g)cinnoline-3-carboxylic Acid, 1-ethyl-1,4-dihydro-4-oxo
162. 5-ethyl-8-oxo-5,8-dihydro-1,3-dioxa-5,6-diaza-cyclopenta[b]naphthalene-7-carboxylic Acid
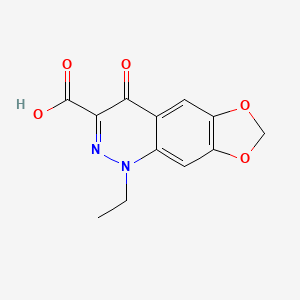
| Molecular Weight | 262.22 g/mol |
|---|---|
| Molecular Formula | C12H10N2O5 |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 2 |
| Exact Mass | 262.05897142 g/mol |
| Monoisotopic Mass | 262.05897142 g/mol |
| Topological Polar Surface Area | 88.4 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 449 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of initial and recurrent urinary tract infections in adults caused by the following susceptible microorganisms: Escherichia coli, Proteus mirabilis, Proteus vulgaris, Klebsiella species (including K. pneumoniae), and Enterobacter species.
FDA Label
Cinoxacin is a synthetic antibacterial agent with in vitro activity against many gram-negative aerobic bacteria, particularly strains of the Enterobacteriaceae family. Cinoxacin inhibits bacterial deoxyribonucleic acid (DNA) synthesis, is bactericidal, and is active over the entire urinary pH range. Cross resistance with nalidixic acid has been demonstrated.
Topoisomerase II Inhibitors
Compounds that inhibit the activity of DNA TOPOISOMERASE II. Included in this category are a variety of ANTINEOPLASTIC AGENTS which target the eukaryotic form of topoisomerase II and ANTIBACTERIAL AGENTS which target the prokaryotic form of topoisomerase II. (See all compounds classified as Topoisomerase II Inhibitors.)
Anti-Infective Agents
Substances that prevent infectious agents or organisms from spreading or kill infectious agents in order to prevent the spread of infection. (See all compounds classified as Anti-Infective Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01M - Quinolone antibacterials
J01MB - Other quinolones
J01MB06 - Cinoxacin
Absorption
Rapidly absorbed after oral administration. While concurrent food intake may delay the drug absorption, the total drug absorption is not affected.
Hepatic, with approximately 30-40% metabolized to inactive metabolites.
While the mean serum half-life is 1.5 hours, the half-life may exceed 10 hours in case of renal impairment.
Evidence exists that cinoxacin binds strongly, but reversibly, to DNA, interfering with synthesis of RNA and, consequently, with protein synthesis. It appears to also inhibit DNA gyrase. This enzyme is necessary for proper replicated DNA separation. By inhibiting this enzyme, DNA replication and cell division is inhibited.
Global Sales Information

ANALYTICAL

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
