

Synopsis
Synopsis
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz

1. Balminil
2. Bay E 5009
3. Bayotensin
4. Baypresol
5. Baypress
6. Gericin
7. Jutapress
8. Nidrel
9. Niprina
10. Nitre Abz
11. Nitre Puren
12. Nitre-puren
13. Nitregamma
14. Nitren 1a Pharma
15. Nitren Acis
16. Nitren Lich
17. Nitrend Ksk
18. Nitrendepat
19. Nitrendi Biochemie
20. Nitrendidoc
21. Nitrendimerck
22. Nitrendipin Al
23. Nitrendipin Apogepha
24. Nitrendipin Atid
25. Nitrendipin Basics
26. Nitrendipin Beta
27. Nitrendipin Corax
28. Nitrendipin Heumann
29. Nitrendipin Jenapharm
30. Nitrendipin Lindo
31. Nitrendipin Ratiopharm
32. Nitrendipin Stada
33. Nitrendipin Von Ct
34. Nitrendipin-corax
35. Nitrendipin-ratiopharm
36. Nitrendipincorax
37. Nitrendipino Bayvit
38. Nitrendipino Ratiopharm
39. Nitrendipinratiopharm
40. Nitrensal
41. Nitrepress
42. Nitrepuren
43. Tensogradal
44. Trendinol
45. Vastensium
1. 39562-70-4
2. Bayotensin
3. Baypress
4. Nidrel
5. Deiten
6. Bay-e-5009
7. Nitrendipinum
8. Nitrepin
9. Bay E 5009
10. Nitrendipino
11. Bylotensin
12. 3-ethyl 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
13. Nitrendipinum [inn-latin]
14. Nitrendipino [inn-spanish]
15. 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic Acid Ethyl Methyl Ester
16. Chebi:7582
17. Nitrendipin
18. Mfcd00082255
19. Nsc-758466
20. Mls000069349
21. 3,5-pyridinedicarboxylic Acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, Ethyl Methyl Ester
22. 5-o-ethyl 3-o-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
23. Ethyl Methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
24. 9b627aw319
25. Ncgc00015713-07
26. Smr000058366
27. Dsstox_cid_3373
28. Dsstox_rid_77001
29. Dsstox_gsid_23373
30. 3,5-pyridinedicarboxylic Acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, Ethyl Methyl Ester, (+/-)-
31. Baylotensin
32. Baypress (tn)
33. 3,5-pyridinedicarboxylic Acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, 3-ethyl 5-methyl Ester
34. Sr-01000075334
35. Einecs 254-513-1
36. Brn 0498823
37. Unii-9b627aw319
38. Nitrendipine,(s)
39. Nitrendipine [usan:inn:ban:jan]
40. Cas-39562-70-4
41. Spectrum_001901
42. Opera_id_928
43. Specplus_000742
44. Prestwick0_000916
45. Prestwick1_000916
46. Prestwick2_000916
47. Prestwick3_000916
48. Spectrum2_001565
49. Spectrum3_000968
50. Spectrum4_001088
51. Spectrum5_001655
52. Nitrendipine [mi]
53. N-144
54. (.+/-.)-nitrendipine
55. Nitrendipine [inn]
56. Nitrendipine [jan]
57. Nitrendipine [usan]
58. (+-)-ethyl Methyl 1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate
59. Ethyl 1,4-dihydro-5-(acetoxycarbonyl)-2,6-dimethyl-4-(3-nitrophenyl)-3-pyridincarboxylat
60. Cbiol_001834
61. Lopac0_000881
62. Oprea1_472855
63. Oprea1_703261
64. Schembl38972
65. Bspbio_000792
66. Bspbio_001399
67. Bspbio_002575
68. Kbiogr_000119
69. Kbiogr_001476
70. Kbioss_000119
71. Kbioss_002432
72. Nitrendipine [mart.]
73. Mls000759400
74. Mls001148149
75. Mls001424133
76. Mls002153303
77. Mls002154060
78. (+/-)-nitrendipine
79. Divk1c_006838
80. Nitrendipine [who-dd]
81. Nitrendipine, >95%, Powder
82. Spectrum1503609
83. Spbio_001470
84. Spbio_002981
85. Bpbio1_000872
86. Gtpl2334
87. Chembl3195219
88. Dtxsid0023373
89. Kbio1_001782
90. Kbio2_000119
91. Kbio2_002426
92. Kbio2_002687
93. Kbio2_004994
94. Kbio2_005255
95. Kbio2_007562
96. Kbio3_000237
97. Kbio3_000238
98. Kbio3_001795
99. Brd6392
100. Nitrendipine (jp17/usan/inn)
101. Bio1_000120
102. Bio1_000609
103. Bio1_001098
104. Bio2_000119
105. Bio2_000599
106. Hms1361f21
107. Hms1570h14
108. Hms1791f21
109. Hms1989f21
110. Hms2051b04
111. Hms2089h15
112. Hms2093g17
113. Hms2097h14
114. Hms2230f04
115. Hms3262b04
116. Hms3266b03
117. Hms3371j07
118. Hms3393b04
119. Hms3402f21
120. Hms3411i14
121. Hms3651f05
122. Hms3675i14
123. Hms3714h14
124. Hms3884h21
125. Nitrendipine [ep Impurity]
126. Pharmakon1600-01503609
127. Nitrendipine [ep Monograph]
128. Bcp07540
129. Brd-6392
130. Hy-b0424
131. Nitrendipine For Peak Identification
132. Tox21_110201
133. Tox21_500881
134. Ac-648
135. Bbl028163
136. Bdbm50237611
137. Ca-212
138. Ccg-39343
139. Nsc758466
140. Stk368903
141. Akos000622913
142. Akos015894921
143. Tox21_110201_1
144. Ccg-100991
145. Db01054
146. Ks-1305
147. Lp00881
148. Nc00241
149. Nsc 758466
150. Sdccgsbi-0050856.p004
151. Idi1_033869
152. Nitrendipine 100 Microg/ml In Methanol
153. Ncgc00015713-04
154. Ncgc00015713-05
155. Ncgc00015713-06
156. Ncgc00015713-08
157. Ncgc00015713-09
158. Ncgc00015713-10
159. Ncgc00015713-11
160. Ncgc00015713-13
161. Ncgc00015713-26
162. Ncgc00024013-02
163. Ncgc00024676-02
164. Ncgc00024676-03
165. Ncgc00024676-04
166. Ncgc00024676-05
167. Ncgc00024676-06
168. Ncgc00024676-07
169. Ncgc00261566-01
170. 3,5-pyridinedicarboxylic Acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, Ethyl Methyl Ester, (+-)-
171. 86052-02-0
172. Sy058769
173. (+/-)-bay-e-5009
174. Sbi-0050856.p003
175. Nitrendipine 100 Microg/ml In Acetonitrile
176. Ab00513962
177. Eu-0100881
178. Ft-0601600
179. N1186
180. S2491
181. Sw219737-1
182. C07713
183. D00629
184. Ab00053154-03
185. Ab00053154_04
186. Ab00053154_05
187. 562n704
188. A824621
189. Q416584
190. Sr-01000075334-1
191. Sr-01000075334-2
192. Sr-01000075334-4
193. Sr-01000075334-6
194. W-106422
195. Brd-a02006392-001-06-5
196. Brd-a02006392-001-09-9
197. Nitrendipine, European Pharmacopoeia (ep) Reference Standard
198. (.+/-.)-ethyl Methyl 1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate
199. 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinecarboxylic Acid Ethyl Methyl Ester
200. 3-ethyl 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydro-3,5-pyridinedicarboxylate #
201. 3-ethyl5-methyl2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
202. Ethyl Methyl 1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate
203. Ethyl Methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydro-3,5-pyridinedicarboxylate
204. Methylethyl2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
205. Nitrendipine For Peak Identification, European Pharmacopoeia (ep) Reference Standard
206. O3-ethyl O5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
207. (+/-)-ethyl Methyl 1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate
208. 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridine Dicarboxylic Acid Ethyl Methyl Ester
209. 3,5-pyridinedicarboxylic Acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, Ethyl Methyl Ester, (.+/-.)-
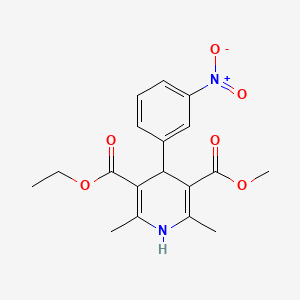
| Molecular Weight | 360.4 g/mol |
|---|---|
| Molecular Formula | C18H20N2O6 |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 6 |
| Exact Mass | 360.13213636 g/mol |
| Monoisotopic Mass | 360.13213636 g/mol |
| Topological Polar Surface Area | 110 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 661 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of mild to moderate hypertension
Nitrendipine, a dihydropyridine calcium-channel blocker, is used alone or with an angiotensin-converting enzyme inhibitor, to treat hypertension, chronic stable angina pectoris, and Prinzmetal's variant angina. Nitrendipine is similar to other peripheral vasodilators. Nitrendipine inhibits the influx of extra cellular calcium across the myocardial and vascular smooth muscle cell membranes possibly by deforming the channel, inhibiting ion-control gating mechanisms, and/or interfering with the release of calcium from the sarcoplasmic reticulum. The decrease in intracellular calcium inhibits the contractile processes of the myocardial smooth muscle cells, causing dilation of the coronary and systemic arteries, increased oxygen delivery to the myocardial tissue, decreased total peripheral resistance, decreased systemic blood pressure, and decreased afterload.
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Calcium Channel Blockers
A class of drugs that act by selective inhibition of calcium influx through cellular membranes. (See all compounds classified as Calcium Channel Blockers.)
C - Cardiovascular system
C08 - Calcium channel blockers
C08C - Selective calcium channel blockers with mainly vascular effects
C08CA - Dihydropyridine derivatives
C08CA08 - Nitrendipine
By deforming the channel, inhibiting ion-control gating mechanisms, and/or interfering with the release of calcium from the sarcoplasmic reticulum, Nitrendipine inhibits the influx of extracellular calcium across the myocardial and vascular smooth muscle cell membranes The decrease in intracellular calcium inhibits the contractile processes of the myocardial smooth muscle cells, causing dilation of the coronary and systemic arteries, increased oxygen delivery to the myocardial tissue, decreased total peripheral resistance, decreased systemic blood pressure, and decreased afterload.
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
