
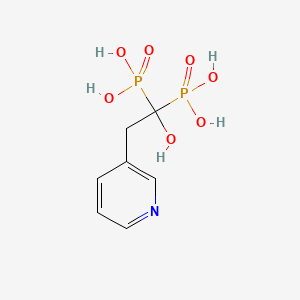

1. 1-hydroxy-2-(3-pyridyl)ethylidene Diphosphonate
2. 2-(3-pyridinyl)-1-hydroxyethylidene-bisphosphonate
3. 2-(3-pyridinyl)-1-hydroxyethylidenebisphosphonate
4. Actonel
5. Atelvia
6. Bisphosphonate Risedronate Sodium
7. Risedronate
8. Risedronate Sodium
9. Risedronate Sodium, Bisphosphonate
10. Risedronic Acid, Monosodium Salt
11. Sodium, Bisphosphonate Risedronate
1. Risedronate
2. 105462-24-6
3. Atelvia
4. Risedronic Acid Monohydrate
5. Ridron
6. Benet
7. Ne-58095
8. (1-hydroxy-1-phosphono-2-pyridin-3-ylethyl)phosphonic Acid
9. [1-hydroxy-1-phosphono-2-(pyridin-3-yl)ethyl]phosphonic Acid
10. M05ba07
11. Risedronic Acid (inn)
12. Risedronic Acid [inn]
13. (1-hydroxy-1-phosphono-2-pyridin-3-yl-ethyl)phosphonic Acid
14. Risedronic Acid (actonel)
15. Chembl923
16. Chebi:8869
17. Phosphonic Acid, (1-hydroxy-2-(3-pyridinyl)ethylidene)bis-
18. Phosphonic Acid, [1-hydroxy-2-(3-pyridinyl)ethylidene]bis-
19. 1-hydroxy-2-(3-pyridinyl)ethylidene Bis-phosphonic Acid
20. Km2z91756z
21. Ris
22. (1-hydroxy-2-pyridin-3-ylethane-1,1-diyl)bis(phosphonic Acid)
23. Acido Risedronico
24. Acide Risedronique
25. Acidum Risedronicum
26. Risedronic Acid [inn:ban]
27. (1-hydroxy-2-(pyridin-3-yl)ethane-1,1-diyl)bis(phosphonic Acid)
28. Acide Risedronique [inn-french]
29. Acido Risedronico [inn-spanish]
30. Acidum Risedronicum [inn-latin]
31. 115436-72-1
32. Bisphosphonate 1
33. [1-hydroxy-1-phosphono-2-(3-pyridyl)ethyl]phosphonic Acid
34. Ridron (tn)
35. Mfcd00867080
36. Ne 58019
37. Hsdb 7326
38. Risdronate
39. Unii-km2z91756z
40. Risedronate Acid
41. Sr-05000001495
42. (1-hydroxy-2-(3-pyridyl)ethylidene)diphosphonic Acid
43. Risedronate [vandf]
44. Schembl18378
45. Risedronic Acid [mi]
46. 2-(3-pyridinyl)-1-hydroxyethane Diphosphonic Acid
47. Bidd:gt0010
48. Risedronic Acid [hsdb]
49. Gtpl3176
50. Jmc515594 Compound 64
51. Dtxsid2023563
52. Risedronic Acid [mart.]
53. Bdbm12576
54. Amy3524
55. Risedronic Acid [who-dd]
56. Hms2090c21
57. Hms3741a17
58. Bcp13805
59. Hy-b0148
60. Zinc1531009
61. S1874
62. Akos015892564
63. Ac-1295
64. Cs-1964
65. Db00884
66. Ncgc00386377-01
67. As-13584
68. Ft-0642593
69. C08233
70. D08484
71. Ab01275490-01
72. Ab01275490_02
73. 462r246
74. A801245
75. Q408724
76. Sr-05000001495-1
77. 2-(3-pyridyl)-1-hydroxyethane-1,1-bisphosphonic Acid
78. Z2684418685
79. (1-hydroxy-2-(pyridin-3-yl)ethane-1,1-diyl)diphosphonic Acid
80. 1-hydroxy-2-(pyridin-3-yl)ethane-1,1-diyldiphosphonic Acid
81. Phosphonic Acid, P,p'-[1-hydroxy-2-(3-pyridinyl)ethylidene]bis-
82. 1-hydroxy-2-(3-pyridinyl)ethylidenebisphosphonicacidmonohydrate;risedronic Acid Monohydrate
| Molecular Weight | 283.11 g/mol |
|---|---|
| Molecular Formula | C7H11NO7P2 |
| XLogP3 | -3.1 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 4 |
| Exact Mass | 283.00107569 g/mol |
| Monoisotopic Mass | 283.00107569 g/mol |
| Topological Polar Surface Area | 148 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 339 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Bone resorption inhibitor.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1478
Risedronate is indicated for the prevention and treatment of glucocorticoid-induction osteoporosis in men and women who are either initiating or continuing systemic glucocorticoid treatment for chronic diseases./Risedronate; Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2475
Risedronate is indicated for the prevention of osteoporosis in postmenopausal women. It may be considered in postmenopausal women who are at risk of developing osteoporosis and for whom the desired clinical outcome is to maintain bone mass and to reduce the risk of fracture. /Risedronate; Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2475
Risedronate is indicated for the treatment of post menopausal osteoporosis. It increases the bone mineral density and reduces the incidence of vertebral fractures and a composite endpoint of nonvertebral osteoporosis fractures. /Risedronate; Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2475
Risedronate is indicated for the treatment of Paget's disease of bone (osteitis deformans) in patients with alkaline phosphatase concentrations that are at least two times the upper limit of normal, those who are symptomatic, or those at risk for future complications from the disease. Signs and symptoms of Paget's disease may include bone pain, deformity, and/or fractures; increased concentrations of N-telopeptide of I collagen, serum alkaline phosphatase, and/or urinary hydroxyproline; neurologic disorders associated with skull lesions and spinal deformities; and elevated cardiac output and other vascular disorders associated with increased vascularity of bones. /Risedronate; Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2475
Risendronate should not be used in patients with severe renal impairment (creatinine clearance less than 30 mL/minute). Adjustments in risedronate sodium dosage are not necessary in patients with mild-to-moderate renal impairment (a creatinine clearance of 30 mL/minute or greater) or in patients with hepatic impairment. /Risedronate/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3617
Adverse upper GI effects (e.g. dysphagia, esophagitis, esophageal or gastric ulcer) have been reported in patients receiving bisphosphonates. In clinical studies, the incidence of such adverse upper GI effects in patients receiving risedronate was similar to that in patients receiving placebo. Data from postmarketing surveillance have occurred, albeit rarely, in patients receiving risedronate sodium 4mg to take risedronate with 180-240 mL of plain water to avoid lying down for 30 minutes following administration of the drug. To minimize risk of adverse upper GI effects, patients should be advised to take risedronate with 180 to 240 mL of plain water and to avoid lying down for 30 minutes following administration of the drug. /Risedronate sodium/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3617
Osteonecrosis and osteomyelitis of the jaws have been reported in patients, principally in these with cancer, who have received bisphosphonates.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3617
Hypocalcemia and other disturbances of bone and mineral metabolism must be corrected before risedronate therapy is initiated, and patients with osteoporosis or Paget's disease of bone should receive supplemental calcium and vitamin D if their daily dietary intake is adequate. /Risedronate/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3617
For more Drug Warnings (Complete) data for RISEDRONIC ACID (10 total), please visit the HSDB record page.
Risedronic acid is indicated for the treatment of osteoperosis in men, treatment of Paget's disease, treatment and prevention of osteoperosis in postmenopausal women, and treatment and prevention of glucocorticoid-induced osteoperosis.
FDA Label
Risedronate is a pyridine-based bisphosphonate that inhibits bone resorption caused by osteoclasts.
Bone Density Conservation Agents
Agents that inhibit BONE RESORPTION and/or favor BONE MINERALIZATION and BONE REGENERATION. They are used to heal BONE FRACTURES and to treat METABOLIC BONE DISEASES such as OSTEOPOROSIS. (See all compounds classified as Bone Density Conservation Agents.)
Calcium Channel Blockers
A class of drugs that act by selective inhibition of calcium influx through cellular membranes. (See all compounds classified as Calcium Channel Blockers.)
M05BA07
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
M - Musculo-skeletal system
M05 - Drugs for treatment of bone diseases
M05B - Drugs affecting bone structure and mineralization
M05BA - Bisphosphonates
M05BA07 - Risedronic acid
Absorption
Oral bioavailability is 0.63% and maximum absorption is approximately 1 hour after dosing. Administration half and hour before a meal reduces bioavailability by 55% compared to fasting and dosing 1 hour before a meal reduces bioavailability by 30%.
Route of Elimination
Risedronate is excreted by the kidneys and the unabsorbed dose is eliminated in the feces.
Volume of Distribution
13.8 L/kg.
Clearance
Mean renal clearance was 52mL/min and mean total clearance was 73mL/min.
/Absorption is/ rapid and independent of dose, occurring throughout the upper gastrointestinal tract. Mean oral bioavailability is 0.63% and is decreased when administered with food. Administration either 0.5 hour before breakfast or 2 hours after dinner reduces the extent of absorption by 55% compared to the fasting state (no food or drink for 10 hours before or 4 hours after administration). Administration 1 hour before breakfast reduces the extent of absorption by 30% compared with the fasting state. /Risedronate/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2475
Studies in rats and dogs with intravenously administered single doses of radiolabeled risedronate showed that approximately 60% of the dose was distributed to bone. The mean steady-state volume of distribution is 6.3 L/kg of body weight in humans. /Risedronate/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2475
After multiple oral dosing in rats, the uptake of risedronate in soft tissues was in the range of 0.001% to 0.01%.
Physicians Desk Reference. 58th ed. Thomson PDR. Montvale, NJ 2004., p. 2825
Risedronate was detected in feeding pups exposed to lactating rats for a 24-hour period postdosing, indicating a small degree of lacteal transfer. /Risedronate/
Physicians Desk Reference. 58th ed. Thomson PDR. Montvale, NJ 2004., p. 2828
Elimination: Fecal, unabsorbed drug (unchanged). Renal, unchanged, approximately 50% of the absorbed dose within 24 hours, 85% over 28 days. Mean renal clearance is 105 mL/minute and mean total clearance is 122 mL/min, the difference primarily reflecting nonrenal clearance or clearance due to absorption to bone. Note: Renal clearance is not concentration dependent and there is a linear relationship between renal clearance and creatinine clearance. /Risedronate/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2475
Risedronic acid is not likely not metabolized before elimination. The P-C-P group of bisphosphonates is resistant to chemical and enzymatic hydrolysis preventing metabolism of the molecule.
There is no evidence that risedronate is metabolized in humans or animals. /Risedronate/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2475
The initial half life of risedronic acid is approximately 1.5 hours, with a terminal half life of 561 hours.
Initial: Approximately 1.5 hours; Terminal exponential: 480 hours (which may represent the dissociation of risedronate from the surface of bone). /Risedronate/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2475
Risedronatic acid binds to bone hydroxyapatite. Bone resorption causes local acidification, releasing risedronic acid which is that taken into osteoclasts by fluid-phase endocytosis. Endocytic vesicles are acidified, releasing risedronic acid to the cytosol of osteoclasts where they induce apoptosis through inhbition of farnesyl pyrophosphate synthase. Inhibition of osteoclasts results in decreased bone resorption.
Risedronate binds to bone hydroxyapatite and, at the cellular level, inhibits osteoclasts. Although the osteoclasts adhere normally to the bone surface, they show evidence of reduced active resorption (e.g., lack of ruffled border). Evidence from studies in rats and dogs indicates that risedronate treatment reduces bone turnover (activation frequency, i.e., the number of sites at which bone is remodeled) and bone resorption at remodeling sites. /Risedronate/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 2475
Risedronate sodium, a synthetic pyridinyl bisphosphonate analog of pyrophosphate, is an inhibitor of osteoclast-mediated bone resorption. / Risedronate sodium/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3618
... Nitrogen-containing bisphosphonates (such as pamidronate, alendronate, risedronate, ibandronate and zoledronate) appear to act as analogues of isoprenoid diphosphate lipids, thereby inhibiting FPP synthase, an enzyme in the mevalonate pathway. Inhibition of this enzyme in osteoclasts prevents the biosynthesis of isoprenoid lipids (FPP and GGPP) that are essential for the post-translational farnesylation and geranylgeranylation of small GTPase signalling proteins. Loss of bone-resorptive activity and osteoclast apoptosis is due primarily to loss of geranylgeranylated small GTPases. Identification of FPP synthase as the target of nitrogen-containing bisphosphonates has also helped explain the molecular basis for the adverse effects of these agents in the GI tract and on the immune system. /Risedronate/
PMID:14529538 Rogers MJ; Curr Pharm Des 9 (32): 2643-58 (2003)
BUILDING BLOCK



LOOKING FOR A SUPPLIER?