

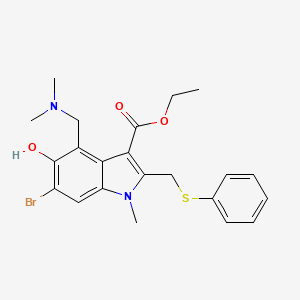

1. 1-methyl-2-((phenylthio)methyl)-3-carbethoxy-4-((dimethylamino)methyl)-5-hydroxy-6-bromoindole
2. 1h-indole-3-carboxylic Acid, 6-bromo-4-((dimethylamino)methyl)-5-hydroxy-1-methyl-2-((phenylthio)methyl)-, Ethyl Ester
3. Arbidole
4. Umifenovir
5. Umifenovir Hydrochloride
6. Umifenovir Hydrochloride Monohydrate
7. Umifenovir Sulfoxide
1. Umifenovir
2. 131707-25-0
3. Umifenovir [inn]
4. Ethyl 6-bromo-4-[(dimethylamino)methyl]-5-hydroxy-1-methyl-2-(phenylsulfanylmethyl)indole-3-carboxylate
5. Ethyl 6-bromo-4-[(dimethylamino)methyl]-5-hydroxy-1-methyl-2-[(phenylsulfanyl)methyl]-1h-indole-3-carboxylate
6. Mls000777586
7. 93m09ww4ru
8. Umifenovir (inn)
9. 1h-indole-3-carboxylic Acid, 6-bromo-4-((dimethylamino)methyl)-5-hydroxy-1-methyl-2-((phenylthio)methyl)-, Ethyl Ester
10. Smr000413980
11. 1h-indole-3-carboxylic Acid, 6-bromo-4-[(dimethylamino)methyl]-5-hydroxy-1-methyl-2-[(phenylthio)methyl]-, Ethyl Ester
12. Unii-93m09ww4ru
13. Arbidol Base
14. 6-bromo-4-dimethylaminomethyl-5-hydroxy-1-methyl-2-phenylsulfanylmethyl-1h-indole-3-carboxylic Acid Ethyl Ester
15. 75u
16. Ethyl 6-bromo-4-((dimethylamino)methyl)-5-hydroxy-1-methyl-2-((phenylsulfanyl)methyl)-1h-indole-3-carboxylate
17. Arbidol; Umifenovir
18. Ar-1i9514
19. Chemdiv1_000732
20. Umifenovir [who-dd]
21. Oprea1_384852
22. Oprea1_482224
23. Mls006011808
24. Cid_131411
25. Schembl1068701
26. Chembl1214598
27. Bdbm83797
28. Gtpl11089
29. Hms589b06
30. Dtxsid60895015
31. Chebi:134730
32. Hms2760j21
33. Albb-028966
34. Bcp04187
35. Ex-a3050
36. Mfcd01326495
37. Stk021887
38. Zinc19907652
39. Akos001485435
40. At13213
41. Db13609
42. Dt-0014
43. Ncgc00246387-01
44. Ncgc00246387-02
45. Ncgc00246387-06
46. Ncgc00246387-09
47. Ethyl 6-bromo-4-((dimethylamino)methyl)-5-hydroxy-1-methyl-2-((phenylthio)methyl)-1h-indole-3-carboxylate
48. Ethyl 6-bromo-4-(dimethylaminomethyl)-5-hydroxy-1-methyl-2-(phenylsulfanylmethyl)indole-3-carboxylate
49. Ethyl 6-bromo-5-hydroxy-1-methyl-4-((dimethylamino)methyl)-2-[(phenylthio)methyl]-1h-indole-3-carboxylate
50. Hy-14904
51. Cs-0003625
52. D10558
53. Ab00644670_06
54. A888382
55. Q27271599
56. 1-methyl-2-((phenylthio)methyl)-3-carbethoxy-4-((dimethylamino)methyl)-5-hydroxy-6-bromindole
57. 1h-indole-3-carboxylic Acid, 6-bromo-4-[(dimethylamino)methyl]-5-hydroxy-1-methyl-2-[(phenylthio)methyl]-, Ethyl Ester, Hydrochloride
58. 5674-91-9
59. 6-bromo-4-[(dimethylamino)methyl]-5-hydroxy-1-methyl-2-[(phenylthio)methyl]-3-indolecarboxylic Acid Ethyl Ester
60. 6-bromo-4-[(dimethylamino)methyl]-5-hydroxy-1-methyl-2-[(phenylthio)methyl]indole-3-carboxylic Acid Ethyl Ester
61. 6-bromo-4-dimethylaminomethyl-5-hydroxy-1-methyl-2-phenylsulfanylmethyl-1h-indole-3-carboxylic Acid Ethyl Ester
62. 6-bromo-5-hydroxy-4-methylaminomethyl-1-methyl-2-benzenesulfenylmethylindole-3-ethyl Carboxylate
63. Ethyl 6-bromanyl-4-[(dimethylamino)methyl]-1-methyl-5-oxidanyl-2-(phenylsulfanylmethyl)indole-3-carboxylate
64. Ethyl 6-bromo-5-hydroxy-4-dimethylaminomethyl-1-methyl-2-phenylthiomethylindole-3-carboxilate
| Molecular Weight | 477.4 g/mol |
|---|---|
| Molecular Formula | C22H25BrN2O3S |
| XLogP3 | 4.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 8 |
| Exact Mass | 476.07693 g/mol |
| Monoisotopic Mass | 476.07693 g/mol |
| Topological Polar Surface Area | 80 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 546 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Umifenovir is currently licensed in China and Russia for the prophylaxis and treatment of influenza and other respiratory viral infections. It has demonstrated activity against a number of viruses and has been investigated in the treatment of _Flavivirus_, Zika virus, foot-and-mouth disease, Lassa virus, Ebola virus, and herpes simplex. In addition, it has shown _in vitro_ activity against hepatitis B and C viruses, chikungunya virus, reovirus, Hantaan virus, and coxsackie virus B5. Umifenovir is currently being investigated as a potential treatment and prophylactic agent for the prevention of COVID-19 caused by SARS-CoV-2 infections.
Umifenovir exerts its antiviral effects via both direct-acting virucidal activity and by inhibiting one (or several) stage(s) of the viral life cycle. Its broad-spectrum of activity covers both enveloped and non-enveloped RNA and DNA viruses. It is relatively well-tolerated and possesses a large therapeutic window - weight-based doses up to 100-fold greater than those used in humans failed to produce any pathological changes in test animals. Umifenovir does not appear to result in significant viral resistance. Instances of umifenovir-resistant influenza virus demonstrated a single mutation in the HA2 subunit of influenza hemagglutinin, suggesting resistance is conferred by prevention of umifenovirs activity related to membrane fusion. The mechanism through which other viruses may become resistant to umifenovir requires further study.
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AX - Other antivirals
J05AX13 - Umifenovir
Absorption
Umifenovir is rapidly absorbed following oral administration, with an estimated Tmax between 0.65-1.8 hours. The Cmax has been estimated as 415 - 467 ng/mL and appears to increase linearly with dose, and the AUC0-inf following oral administration has been estimated to be approximately 2200 ng/mL/h.
Route of Elimination
The major route of elimination is via the feces. Approximately 40% of an ingested dose is excreted unchanged, of which 38.9% is excreted in the bile and 0.12% excreted through the kidneys. The total recovery of parent drug and metabolites in the urine accounts for less than 1% of an ingested dose.
Volume of Distribution
Data regarding the volume of distribution of umifenovir are currently unavailable.
Clearance
In a study involving healthy male Chinese volunteers, the oral clearance of umifenovir was found to be 99 34 L/h.
Umifenovir is highly metabolized in the body, primarily in hepatic and intestinal microsomess, with approximately 33 metabolites having been observed in human plasma, urine, and feces. The principal phase I metabolic pathways include sulfoxidation, N-demethylation, and hydroxylation, followed by phase II sulfate and glucuronide conjugation. In the urine, the major metabolites were sulfate and glucuronide conjugates, while the major species in the feces was unchanged parent drug (~40%) and the M10 metabolite (~3.0%). In the plasma, the principal metabolites are M6-1, M5, and M8 - of these, M6-1 appears of most importance given its high plasma exposure and long elimination half-life (~25h), making it a potentially important player in the safety and efficacy of umifenovir. Enzymes involved in the metabolism of umifenovir include members of the cytochrome P450 family (primarily CYP3A4), flavin-containing monooxygenase (FMO) family, and UDP-glucuronosyltransferase (UGT) family (specifically UGT1A9 and UGT2B7).
Arbidol has known human metabolites that include (2S,3S,4S,5R)-6-[6-bromo-4-[(dimethylamino)methyl]-3-ethoxycarbonyl-1-methyl-2-(phenylsulfanylmethyl)indol-5-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The half-life of umifenovir following oral administration has been estimated to be between 17-21 hours. Serum half-lives of the M5, M6-1, and M8 metabolites were found to be 26.3 5.9, 25.0 5.4, and 25.7 8.8, respectively.
Umifenovir is considered both a direct-acting antiviral (DAA) due to direct virucidal effects and a host-targeting agent (HTA) due to effects on one or multiple stages of viral life cycle (e.g. attachment, internalization), and its broad-spectrum antiviral activity is thought to be due to this dual activity. It is a hydrophobic molecule capable of forming aromatic stacking interactions with certain amino acid residues (e.g. tyrosine, tryptophan), which contributes to its ability to directly act against viruses. Antiviral activity may also be due to interactions with aromatic residues within the viral glycoproteins involved in fusion and cellular recognition, with the plasma membrane to interfere with clathrin-mediated exocytosis and intracellular trafficking, or directly with the viral lipid envelope itself (in enveloped viruses). Interactions at the plasma membrane may also serve to stabilize it and prevent viral entry (e.g. stabilizing influenza hemagglutinin inhibits the fusion step necessary for viral entry). Due to umifenovirs ability to interact with both viral proteins and lipids, it may also interfere with later stages of the viral life cycle. Some virus families, such as _Flaviviridae_, replicate in a subcellular compartment called the membranous web - this web requires lipid-protein interactions that may be hindered by umifenovir. Similarly, viral assembly of hepatitis C viruses is contingent upon the assembly of lipoproteins, presenting another potential target.

LOOKING FOR A SUPPLIER?