

Synopsis
Synopsis
0
JDMF
0
KDMF
0
VMF
0
API
0
Canada

0
Australia

0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
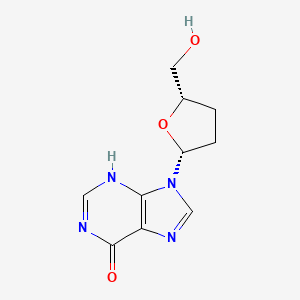

| Molecular Weight | 236.23 g/mol |
|---|---|
| Molecular Formula | C10H12N4O3 |
| XLogP3 | -1.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 236.09094026 g/mol |
| Monoisotopic Mass | 236.09094026 g/mol |
| Topological Polar Surface Area | 88.7 A^2 |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 348 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Didanosine |
| Drug Label | Didanosine delayed-release capsules are an enteric-coated formulation of didanosine, (ddl), a synthetic purine nucleoside analogue active against the Human Immunodeficiency Virus (HIV). Didanosine delayed-release capsules, containing enteric-coated p... |
| Active Ingredient | Didanosine |
| Dosage Form | Capsule, delayed rel pellets; Tablet, for suspension; For solution |
| Route | Oral |
| Strength | 200mg; 250mg; 100mg; 125mg; 150mg; 10mg/ml; 400mg |
| Market Status | Prescription |
| Company | Mylan Pharms; Aurobindo; Aurobindo Pharma; Barr |
| 2 of 6 | |
|---|---|
| Drug Name | Videx |
| PubMed Health | Didanosine (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | VIDEX is a brand name for didanosine, USP, a synthetic purine nucleoside analogue active against HIV-1. Didanosine is available as VIDEX, a Pediatric Powder for Oral Solution [see How Supplied/Storage and Handling (16)] and as VIDEX EC Delayed-Re... |
| Active Ingredient | Didanosine |
| Dosage Form | For solution |
| Route | Oral |
| Strength | 10mg/ml |
| Market Status | Prescription |
| Company | Bristol Myers Squibb |
| 3 of 6 | |
|---|---|
| Drug Name | Videx ec |
| PubMed Health | Didanosine (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | VIDEX EC is the brand name for an enteric-coated formulation of didanosine, USP, a synthetic purine nucleoside analogue active against HIV-1. VIDEX EC Delayed-Release Capsules, containing enteric-coated beadlets, are available for oral administrati... |
| Active Ingredient | Didanosine |
| Dosage Form | Capsule, delayed rel pellets |
| Route | Oral |
| Strength | 200mg; 250mg; 125mg; 400mg |
| Market Status | Prescription |
| Company | Bristol Myers Squibb |
| 4 of 6 | |
|---|---|
| Drug Name | Didanosine |
| Drug Label | Didanosine delayed-release capsules are an enteric-coated formulation of didanosine, (ddl), a synthetic purine nucleoside analogue active against the Human Immunodeficiency Virus (HIV). Didanosine delayed-release capsules, containing enteric-coated p... |
| Active Ingredient | Didanosine |
| Dosage Form | Capsule, delayed rel pellets; Tablet, for suspension; For solution |
| Route | Oral |
| Strength | 200mg; 250mg; 100mg; 125mg; 150mg; 10mg/ml; 400mg |
| Market Status | Prescription |
| Company | Mylan Pharms; Aurobindo; Aurobindo Pharma; Barr |
| 5 of 6 | |
|---|---|
| Drug Name | Videx |
| PubMed Health | Didanosine (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | VIDEX is a brand name for didanosine, USP, a synthetic purine nucleoside analogue active against HIV-1. Didanosine is available as VIDEX, a Pediatric Powder for Oral Solution [see How Supplied/Storage and Handling (16)] and as VIDEX EC Delayed-Re... |
| Active Ingredient | Didanosine |
| Dosage Form | For solution |
| Route | Oral |
| Strength | 10mg/ml |
| Market Status | Prescription |
| Company | Bristol Myers Squibb |
| 6 of 6 | |
|---|---|
| Drug Name | Videx ec |
| PubMed Health | Didanosine (By mouth) |
| Drug Classes | Antiretroviral Agent |
| Drug Label | VIDEX EC is the brand name for an enteric-coated formulation of didanosine, USP, a synthetic purine nucleoside analogue active against HIV-1. VIDEX EC Delayed-Release Capsules, containing enteric-coated beadlets, are available for oral administrati... |
| Active Ingredient | Didanosine |
| Dosage Form | Capsule, delayed rel pellets |
| Route | Oral |
| Strength | 200mg; 250mg; 125mg; 400mg |
| Market Status | Prescription |
| Company | Bristol Myers Squibb |
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
