

Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
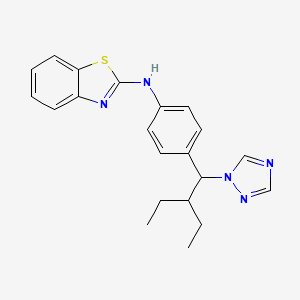

1. N-(4-(2-ethyl-1-(1h-1,2,4-triazol-1-yl)butyl)phenyl)-2-benzothiazolamine
2. R 115866
3. R-115866
4. R115866
5. Rambazole
1. 201410-53-9
2. Rambazole
3. R115866
4. R-115866
5. N-(4-(2-ethyl-1-(1h-1,2,4-triazol-1-yl)butyl)phenyl)benzo[d]thiazol-2-amine
6. Talarozole (r Enantiomer)
7. Xkd9n5cj6w
8. 201410-66-4
9. N-[4-[2-ethyl-1-(1,2,4-triazol-1-yl)butyl]phenyl]-1,3-benzothiazol-2-amine
10. Chembl459505
11. (+)-n-[4-[2-ethyl-1-(1h-1,2,4-triazol-1-yl)butyl]phenyl]-2-benzothiazolamine
12. 2-benzothiazolamine, N-[4-[2-ethyl-1-(1h-1,2,4-triazol-1-yl)butyl]phenyl]-
13. N-(4-((1rs)-2-ethyl-1-(1h-1,2,4-triazol-1-yl)butyl)phenyl)benzothiazol-2-amine
14. Rambazole (tn)
15. Talarozole [usan]
16. Talarozole (usan/inn)
17. Talarozole [usan:inn]
18. Unii-xkd9n5cj6w
19. 2-benzothiazolamine, N-(4-(2-ethyl-1-(1h-1,2,4-triazol-1-yl)butyl)phenyl)-
20. N-{4-[2-ethyl-1-(1,2,4-triazol-1-yl)butyl]phenyl}-1,3-benzothiazol-2-amine
21. R 115866
22. N-(4-(2-ethyl-1-(1h-1,2,4-triazol-1-yl)butyl)phenyl)-2-benzothiazolamine
23. Talarozole [inn]
24. Talarozole [who-dd]
25. Schembl721201
26. Schembl21020998
27. Gtpl11381
28. Dtxsid70942185
29. Chebi:102167
30. Bcp21218
31. Bcp28256
32. Bia41053
33. Bdbm50253810
34. Akos005067289
35. Cs-1343
36. Db13083
37. Ncgc00378894-01
38. Ncgc00378894-02
39. Hy-14531
40. Ws-02133
41. D09385
42. D85497
43. A926099
44. Q15410180
45. Rambazole; R115866; R-115866; R 115866
46. Talarozole R Enantiomer;r115866;r 115866;r-115866
47. R115866;r-115866;r 115866
48. (+/-)-n-[4-[2-ethyl-1-(1h-1,2,4-triazol-1-yl)butyl]phenyl]-2-benzothiazol-amine
49. (+/-)-n-[4-[2-ethyl-1-(1h-1,2,4-triazol-1-yl)butyl]phenyl]-2-benzothiazolamine
50. N-{4-[2-ethyl-1-(1h-1,2,4-triazol-1-yl)butyl]phenyl}-1,3-benzothi Azol-2-amine
51. N-{4-[2-ethyl-1-(1h-1,2,4-triazol-1-yl)butyl]phenyl}-1,3-benzothiazol-2-amine
| Molecular Weight | 377.5 g/mol |
|---|---|
| Molecular Formula | C21H23N5S |
| XLogP3 | 6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 7 |
| Exact Mass | 377.16741693 g/mol |
| Monoisotopic Mass | 377.16741693 g/mol |
| Topological Polar Surface Area | 83.9 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 452 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Cytochrome P-450 Enzyme Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 ENZYMES. (See all compounds classified as Cytochrome P-450 Enzyme Inhibitors.)
Rambazole is a new generation all-trans retinoic acid metabolism blocking agent, highly specific against the retinoic acid 4-hydroxylase. The drug alleviates hyperproliferation and normalizes differentiation of the epidermis in animal models of psoriasis. All-trans-retinoic acid (RA) regulates epithelial differentiation and growth through activation of specific nuclear RA receptors (RARs). Rambazole acts by inhibiting the metabolic breakdown of the retinoid, increasing the biological efficacy of RA.
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
