
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
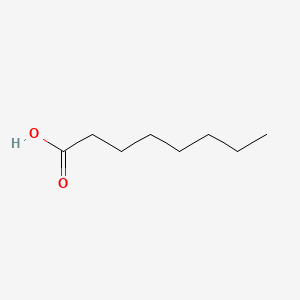

1. Caprylic Acid
2. Caprylic Acid, 14c-labeled
3. Caprylic Acid, Aluminum Salt
4. Caprylic Acid, Ammonia Salt
5. Caprylic Acid, Barium Salt
6. Caprylic Acid, Cadmium Salt
7. Caprylic Acid, Calcium Salt
8. Caprylic Acid, Cesium Salt
9. Caprylic Acid, Chromium(+2) Salt
10. Caprylic Acid, Cobalt Salt
11. Caprylic Acid, Copper Salt
12. Caprylic Acid, Copper(+2) Salt
13. Caprylic Acid, Iridum(+3) Salt
14. Caprylic Acid, Iron(+3) Salt
15. Caprylic Acid, Lanthanum(+3) Salt
16. Caprylic Acid, Lead(+2) Salt
17. Caprylic Acid, Lithium Salt
18. Caprylic Acid, Manganese Salt
19. Caprylic Acid, Nickel(+2) Salt
20. Caprylic Acid, Potassium Salt
21. Caprylic Acid, Ruthenium(+3) Salt
22. Caprylic Acid, Sodium Salt
23. Caprylic Acid, Sodium Salt, 11c-labeled
24. Caprylic Acid, Tin Salt
25. Caprylic Acid, Tin(+2) Salt
26. Caprylic Acid, Zinc Salt
27. Caprylic Acid, Zirconium Salt
28. Caprylic Acid, Zirconium(+4) Salt
29. Lithium Octanoate
30. Octanoate
31. Sodium Caprylate
32. Sodium Octanoate
1. Caprylic Acid
2. 124-07-2
3. N-octanoic Acid
4. Octylic Acid
5. N-caprylic Acid
6. Octoic Acid
7. N-octylic Acid
8. N-octoic Acid
9. 1-heptanecarboxylic Acid
10. Neo-fat 8
11. Enantic Acid
12. Octic Acid
13. C-8 Acid
14. Fema No. 2799
15. Kaprylsaeure
16. Hexacid 898
17. Acido Octanoico
18. Acide Octanoique
19. 1-octanoic Acid
20. Acidum Octanocium
21. Fatty Acids, C6-10
22. Kyselina Kaprylova
23. Capryloate
24. C8:0
25. Octylate
26. Octansaeure
27. Nsc 5024
28. Nsc-5024
29. Octanoic Acid (caprylic Acid)
30. Kortacid-0899
31. Chebi:28837
32. Emery 657
33. Prifac 2901
34. Prifac-2901
35. Lunac 8-95
36. Edenor C 8-98-100
37. Ch3-[ch2]6-cooh
38. Obl58jn025
39. Caprylsaeure
40. Nsc5024
41. N-caprylate
42. N-octoate
43. N-octylate
44. Caprylic Acid (nf)
45. 68937-74-6
46. Ncgc00090957-01
47. Octanoic Acid (usan)
48. 0ctanoic Acid
49. 1-heptanecarboxylate
50. Dsstox_cid_1645
51. Caprylic Acid [nf]
52. Dsstox_rid_76259
53. Dsstox_gsid_21645
54. Octanoic Acid [usan]
55. Caprylic Acid (natural)
56. Acide Octanoique [french]
57. Acido Octanoico [spanish]
58. Acidum Octanocium [latin]
59. Kyselina Kaprylova [czech]
60. Octanoic Acid [usan:inn]
61. 287111-06-2
62. Cas-124-07-2
63. Acid C8
64. Ccris 4689
65. Hsdb 821
66. Einecs 204-677-5
67. Mfcd00004429
68. Brn 1747180
69. Unii-obl58jn025
70. Caprylic-acid
71. N-octanoicacid
72. Octanic Acid
73. Ai3-04162
74. Acidum Octanoicum
75. Einecs 273-085-7
76. Kortacid 0899
77. Neo-fat 8s
78. Caprylic Acid 657
79. N-heptanecarboxylic Acid
80. Fatty Acids, C6-1o
81. Lunac 8-98
82. Heptane-1-carboxylic Acid
83. Octanoic Acid, >=98%
84. Octanoic Acid, >=99%
85. Bmse000502
86. Caprylic/capric Acid Blend
87. Ec 204-677-5
88. Octanoic Acid-2-[13c]
89. Caprylic Acid [mi]
90. Octanoic Acid [ii]
91. Schembl3933
92. Wln: Qv7
93. Nciopen2_002902
94. Nciopen2_009358
95. Octanoic Acid (usan/inn)
96. Octanoic Acid [inn]
97. Caprylic Acid [inci]
98. Octanoic Acid [fhfi]
99. Octanoic Acid [hsdb]
100. 4-02-00-00982 (beilstein Handbook Reference)
101. Mls002415762
102. Octanoic Acid, >=96.0%
103. Caprylic Acid (octanoic Acid)
104. Caprylic Acid [vandf]
105. Octanoic Acid (mixed Isomers)
106. Octanoic Acid [mart.]
107. Chembl324846
108. Gtpl4585
109. Octanoic Acid, >=98%, Fg
110. Qspl 011
111. Qspl 184
112. Caprylic Acid [usp-rs]
113. Octanoic Acid [who-dd]
114. Dtxsid3021645
115. Octanoic Acid-1,2-[13c2]
116. Octanoic Acid-7,8-[13c2]
117. Hms2270a23
118. Octanoic Acid, Analytical Standard
119. Caprylic Acid [ep Impurity]
120. Str10050
121. Zinc1530416
122. Tox21_111045
123. Tox21_201279
124. Tox21_300345
125. Bdbm50485608
126. Caprylic Acid [ep Monograph]
127. Lmfa01010008
128. S6296
129. Stl282742
130. Akos000118802
131. Octanoic Acid, Natural, >=98%, Fg
132. Db04519
133. Fa(8:0)
134. Octanoic Acid, For Synthesis, 99.5%
135. Ncgc00090957-02
136. Ncgc00090957-03
137. Ncgc00090957-04
138. Ncgc00090957-05
139. Ncgc00254446-01
140. Ncgc00258831-01
141. Bp-27909
142. Hy-41417
143. Smr001252279
144. Cs-0016549
145. Ft-0660765
146. O0027
147. Octanoic Acid 100 Microg/ml In Acetonitrile
148. C06423
149. D05220
150. Q409564
151. Sr-01000865607
152. J-005040
153. Sr-01000865607-2
154. Brd-k35170555-001-07-9
155. Z955123584
156. Caprylic Acid (constituent Of Saw Palmetto) [dsc]
157. Octanoic Acid, Certified Reference Material, Tracecert(r)
158. 43fda9d7-2300-41e7-a373-a34f25b81553
159. Caprylic Acid, European Pharmacopoeia (ep) Reference Standard
160. Caprylic Acid, United States Pharmacopeia (usp) Reference Standard
161. Caprylic Acid (octanoic Acid), Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 144.21 g/mol |
|---|---|
| Molecular Formula | C8H16O2 |
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 6 |
| Exact Mass | 144.115029749 g/mol |
| Monoisotopic Mass | 144.115029749 g/mol |
| Topological Polar Surface Area | 37.3 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 89.3 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Exptl Use: A simple methodology for hyperimmune horse plasma fractionation, based on caprylic acid precipitation, is described. Optimal conditions for fractionation were studied; the method gives best results when concentrated caprylic acid was added to plasma, whose pH had been adjusted to 5.8, until a final caprylic acid concentration of 5% was reached. The mixture was vigorously stirred during caprylic acid addition and then for 60 min; afterwards the mixture was filtered. Non-immunoglobulin proteins precipitated in these conditions, whereas a highly enriched immunoglobulin preparation was obtained in the filtrate, which was then dialysed to remove caprylic acid before the addition of sodium chloride and phenol. Thus, antivenon was produced after a single precipitation step followed by dialysis. In order to compare this methodology with that based on ammonium sulfate fractionation, a sample of hyperimmune plasma was divided into two aliquots which were fractionated in parallel by both methods. It was found that caprylic acid-fractionated antivenom was superior in terms of yield, production time, albumin/globulin ratio, turbidity, protein aggregates, electrophoretic pattern and neutralizing potency against several activities of Bothrops asper venom. Owing to its efficacy and simplicity, this method could be of great value in antivenom and antitoxin production laboratories.
PMID:8016856 Rojas G et al; Toxicon 32 (3): 351-63 (1994)
/EXPL THER/ The treatment for patients with genetic disorders of mitochondrial long-chain fatty acid beta-oxidation is directed toward providing sufficient sources of energy for normal growth and development, and at the same time preventing the adverse effects that precipitate or result from metabolic decompensation. Standard of care treatment has focused on preventing the mobilization of lipids that result from fasting and providing medium-chain triglycerides (MCT) in the diet in order to bypass the long-chain metabolic block. MCTs that are currently available as commercial preparations are in the form of even-chain fatty acids that are predominately a mixture of octanoate and decanoate ... The even-numbered medium-chain fatty acids (MCFAs) that are found in MCT preparations can reduce the accumulation of potentially toxic long-chain metabolites of fatty acid oxidation (FAO) ... /Octanoate/
PMID:14741189 Jones PM et al; Mol Genet Metab 81(2):96-9 (2004)
Children who suffer from seizures which are not controllable by drugs have apparently been successfully treated with MCT (medium chain triglyceride) diet. The MCT diet is an emulsion containing primarily (81%) octanoic acid, but also contains 15% decanoic acid ... /Medium chain triglyceride/
European Chemicals Bureau; IUCLID Dataset, Decanoic acid (CAS #334-48-5) p.45 (2000 CD-ROM edition). Available from, as of January 23, 2008: https://esis.jrc.ec.europa.eu/
Children who suffer from seizures which are not controllable by drugs have apparently been successfully treated with MCT (medium chain triglyceride) diet. The MCT diet is an emulsion containing primarily (81%) octanoic acid, but also contains 15% decanoic acid. In this study 15 children were receiving 50 to 60% of their energy requirement s from the MCT emulsion. Blood samples were analyzed for decanoic and octanoic acid levels. There was a wide variation in absolute levels, possibly due to poor patient compliance, but all patients showed low levels in the mornings, rising to high levels in the evenings. This suggested that both acids are rapidly metabolized. /Medium chain triglyceride/
European Chemicals Bureau; IUCLID Dataset, Decanoic acid (CAS #334-48-5) p.45 (2000 CD-ROM edition). Available from, as of January 23, 2008: https://esis.jrc.ec.europa.eu/
To assess the disposition kinetics of selected structural analogs of valproic acid, the pharmacokinetics of valproic acid and 3 structural analogs, cyclohexanecarboxylic acid, l-methyl-l-cyclohexanecarboxylic acid (1-methylcyclohexanecarboxylic acid; and octanoic acid were examined in female rats. All 4 carboxylic acids evidenced dose-dependent disposition. A dose-related decrease in total body clearance was observed for each compound, suggesting saturable eiminination processes. The apparent volume of distribution for these compounds was, with the exception of cyclohexanecarboxylic acid, dose-dependent, indicating that binding to proteins in serum and/or tissues may be saturable. Both valproic acid and 1-methylcyclohexanecarboxylic acid exhibited enterohepatic recirculation, which appeared to be dose- and compound-dependent. Significant quantities of both valproic acid and 1-methylcyclohexanecarboxylic acid were excreted in the urine as conjugates. Octanoic acid and cyclohexanecarboxylic acid were not excreted in the urine and did not evidence enterohepatic recirculation. It was concluded that minor changes in chemical structure of low molecular weight carboxylic acids have an influence on their metabolism and disposition.
PMID:8499583 Liu MJ, Pollack GM; Biopharm Drug Dispos 14 (May): 325-39 (1993)
Caprylic acid administered to rats is readily metabolized by the liver and many other tissues, forming carbon dioxide and two-carbon fragments, which are incorporated into long-chain fatty acids, as well as other water-soluble products.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. 726
Mitochondrial uncoupling is often invoked as a mechanism underlying cellular dysfunction; however, it has not been possible to study this phenomenon directly in intact cells and tissues. In this paper ... direct evaluation of mitochondrial uncoupling in the intact myocardium using (31)P NMR magnetization transfer techniques /are reported/. Langendorff perfused rat hearts were exposed to either a known uncoupler, 2,4-dinitrophenol, or a potential uncoupler, octanoate. Both 2,4-dinitrophenol and octanoate decreased mechanical function as measured by the rate pressure product and caused an increase in the oxygen consumption rate; with 2,4-dinitrophenol this increase in oxygen consumption rate was dose-dependent. The ATP synthesis rate measured by (31)P NMR, however, was not elevated commensurately with oxygen consumption rate; instead, the P/O ratio declined. In contrast, the linear relationship between the ATP synthesis rate and rate pressure product was not altered by the uncoupling agents. These data demonstrate that 1) (31)P NMR magnetization transfer can be utilized to measure uncoupling of oxidative phosphorylation in intact organs, 2) octanoate does not induce excess ATP utilization in the intact heart, and 3) high levels of octanoate induce mitochondrial uncoupling in the intact myocardium; and this may, in part, be the cause of the toxic effects associated with fatty acid exposure. /Octanoate/
PMID:2136855 Kingsley-Hickman PB et al; J Biol Chem 265 (3): 1545-50 (1990)
It has been shown that polyunsaturated fatty acids such as arachidonic and docosahexanoic acids but not monounsaturated and saturated long-chain fatty acids promote basal and nerve growth factor (NGF)-induced neurite extension of PC12 cells, a line derived from a rat pheochromocytoma. On the other hand, short-chain fatty acids and valproic acid (2-propylpentanoic acid) enhance the growth of neurite processes of the cells only in the presence of inducers. In this study, /investigators/ demonstrated that straight medium-chain fatty acids (MCFAs) at millimolar concentrations alone potently induced neuronal differentiation of PC12 cells. ... Nonanoic, decanoic, and dodecanoic acids also induced growth of neurite processes, but their maximal effects were less marked than that of octanoic acid. ...
PMID:17434686 Kamata Y et al; Neuroscience 146 (3): 1073-81 (2007)

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?