

Synopsis
Synopsis
0
EU WC
0
KDMF
0
VMF
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
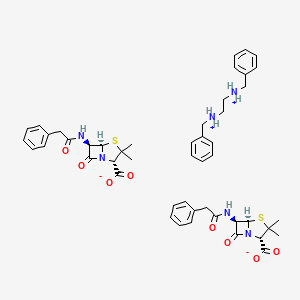

1. Bencelin
2. Benzathine Benzylpnicilline Panpharma
3. Benzathine Benzylpenicillin
4. Benzathine Penicillin
5. Benzathine, Penicillin G
6. Benzetacil
7. Benzylpenicillin, Benzathine
8. Bicillin
9. Bicillin L A
10. Bicillin La
11. Brevicilina
12. Cepacilina
13. Debecillin
14. Extencilline
15. Pendepon
16. Penduran
17. Pendysin
18. Penicillin G Benzathine
19. Penicillin G Benzathine Anhydrous
20. Penicillin, Benzathine
21. Penidural
22. Peniroger Retard
23. Permapen
24. Provipen Benzatina
25. Tardocillin
1. Benzathine Benzylpenicillin
2. Cepacilina
3. Lentopenil
4. Tardocillin
5. Beacillin
6. Penidural
7. Chebi:51352
8. Benzylpenicillin Dibenzylethylenediamine Salt
9. Penicillin G Salt Of N,n'-dibenzylethylenediamine
10. N,n'-dibenzylethylenediamine Bis(benzyl Penicillin)
11. Epitope Id:224559
12. Db09323
13. N,n'-dibenzylethane-1,2-diaminium Bis{2,2-dimethyl-6beta-(phenylacetamido)penam-3alpha-carboxylate}
| Molecular Weight | 909.1 g/mol |
|---|---|
| Molecular Formula | C48H56N6O8S2 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 13 |
| Exact Mass | 908.36010511 g/mol |
| Monoisotopic Mass | 908.36010511 g/mol |
| Topological Polar Surface Area | 263 Ų |
| Heavy Atom Count | 64 |
| Formal Charge | 0 |
| Complexity | 696 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
| 1 of 2 | |
|---|---|
| Drug Name | Bicillin l-a |
| PubMed Health | Penicillin G Benzathine (Injection) |
| Drug Classes | Antibiotic |
| Drug Label | Bicillin L-A (penicillin G benzathine injectable suspension) is available for deep intramuscular injection. Penicillin G benzathine is prepared by the reaction of dibenzylethylene diamine with two molecules of penicillin G. It is chemically designate... |
| Active Ingredient | Penicillin g benzathine |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 300,000 units/ml; 600,000 units/ml |
| Market Status | Prescription |
| Company | King Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Bicillin l-a |
| PubMed Health | Penicillin G Benzathine (Injection) |
| Drug Classes | Antibiotic |
| Drug Label | Bicillin L-A (penicillin G benzathine injectable suspension) is available for deep intramuscular injection. Penicillin G benzathine is prepared by the reaction of dibenzylethylene diamine with two molecules of penicillin G. It is chemically designate... |
| Active Ingredient | Penicillin g benzathine |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 300,000 units/ml; 600,000 units/ml |
| Market Status | Prescription |
| Company | King Pharms |
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01C - Beta-lactam antibacterials, penicillins
J01CE - Beta-lactamase sensitive penicillins
J01CE08 - Benzathine benzylpenicillin
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 13655
Submission : 1998-09-01
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 13318
Submission : 1998-09-01
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2018-04-19
Pay. Date : 2018-03-23
DMF Number : 13294
Submission : 1998-09-01
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 13328
Submission : 1998-09-01
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Benzylpenicillin (benzathine) tetrahydrate, Sterile, microfine, lecithin/polysorbate coated
Certificate Number : R1-CEP 2001-066 - Rev 05
Status : Valid
Issue Date : 2023-07-26
Type : Chemical
Substance Number : 373

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 1994-018 - Rev 04
Status : Withdrawn by Holder
Issue Date : 2004-12-01
Type : Chemical
Substance Number : 373


Benzylpenicillin (benzathine) tetrahydrate, Lecithin and Polysorbate 80 coated, Sterile
Certificate Number : R1-CEP 2002-111 - Rev 01
Status : Withdrawn by Holder
Issue Date : 2009-10-22
Type : Chemical
Substance Number : 373

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
