
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
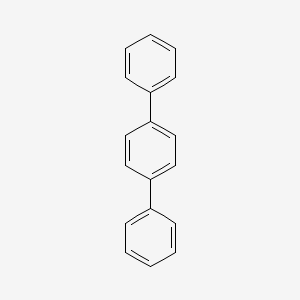

1. 92-94-4
2. 1,4-diphenylbenzene
3. 1,1':4',1''-terphenyl
4. P-diphenylbenzene
5. P-triphenyl
6. Santowax P
7. 4-phenylbiphenyl
8. 4-phenyldiphenyl
9. Para-terphenyl
10. Biphenyl, 4-phenyl-
11. 1,1'-biphenyl, 4-phenyl-
12. Nsc 6810
13. Ptp
14. 4-phenyl-1,1'-biphenyl
15. Gwp218zy6f
16. Chembl491582
17. Chebi:52242
18. Nsc-6810
19. Pyrogallol Tannin
20. Tannin From Pyrogallol
21. P-terphenyl Suitable For Scintillation
22. Ccris 1657
23. Hsdb 5280
24. Einecs 202-205-2
25. Unii-gwp218zy6f
26. Triphenyl-
27. Ai3-00847
28. [1,1';4',1'']terphenyl
29. Ppp (scintillator)
30. Mfcd00003061
31. Tannin Pyrogallol
32. Terphenyl, P-
33. P-phenylene Trimer
34. Dsstox_cid_7888
35. Wln: Rr Dr
36. Dsstox_rid_78673
37. Dsstox_gsid_29121
38. 1,1':4',1"-terphenyl
39. Dtxsid6029121
40. P-terphenyl, Analytical Standard
41. Nsc6810
42. Zinc1867001
43. Tox21_202759
44. Bdbm50260180
45. P-terphenyl, >=99.5% (hplc)
46. Stl069547
47. P-terphenyl (purified By Sublimation)
48. Akos005111366
49. 1,1'':4'',1''''-terphenyl
50. Cs-w014689
51. Cas-92-94-4
52. Ncgc00164113-01
53. Ncgc00260306-01
54. Ac-18695
55. As-12803
56. Db-038209
57. Ft-0659947
58. T0020
59. T3263
60. D92686
61. T-3203
62. 003t061
63. A844395
64. Ab-131/40897106
65. W-100265
66. Q20965188
67. F0486-1779
68. Z1262246140
69. P-terphenyl, Suitable For Scintillation, >=98.5% (hplc)
| Molecular Weight | 230.3 g/mol |
|---|---|
| Molecular Formula | C18H14 |
| XLogP3 | 5.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 2 |
| Exact Mass | 230.109550447 g/mol |
| Monoisotopic Mass | 230.109550447 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 198 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
...POORLY ABSORBED & LARGELY EXCRETED UNCHANGED IN FECES.
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-105
Depending on the extent to which these compounds are absorbed, the various terphenyls were metabolized to glucuronic acid conjugates and phenol and excreted in the urine of treated rabbits. Following a single 1 gram oral dose of o-, m-, or p-terphenyl, 60% of the o-, 38% of the m-, and a trace of p-terphenyl were detected as their respective glucuronides in the urine. Zero, 15%, and 30% of the o-, m-, and p-terphenyls, respectively, were isolated unchanged in feces.
American Conference of Governmental Industrial Hygienists, Inc. Documentation of the Threshold Limit Values and Biological Exposure Indices. 6th ed. Volumes I, II, III. Cincinnati, OH: ACGIH, 1991., p. 1500
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
