

Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
API
0
FDF
0
Australia

0
South Africa

0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
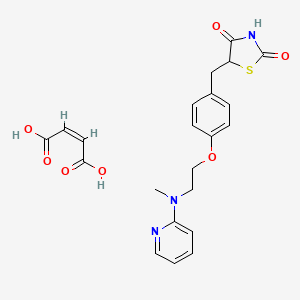

1. 5-((4-(2-methyl-2-(pyridinylamino)ethoxy)phenyl)methyl)-2,4-thiazolidinedione-2-butenedioate
2. Avandia
3. Brl 49653
4. Brl-49653
5. Brl49653
6. Rosiglitazone
1. 155141-29-0
2. Avandia
3. Rosiglitazone (maleate)
4. Rosiglitazone Maleate [usan]
5. Brl-49653c
6. Brl 49653c
7. Rosiglitzazone Maleate
8. 5-(4-(2-(methyl(pyridin-2-yl)amino)ethoxy)benzyl)thiazolidine-2,4-dione Maleate
9. Brl-49653-c
10. Nsc-717764
11. Kx2339dp44
12. Avandamet
13. 2,4-thiazolidinedione, 5-((4-(2-(methyl-2-pyridinylamino)ethoxy)phenyl)methyl)-, (2z)-2-butenedioate (1:1)
14. Nyracta
15. Rosiglitazone Xr
16. Venvia
17. Chebi:8892
18. Brl 49653-c
19. (2z)-but-2-enedioic Acid; 5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)methyl]-1,3-thiazolidine-2,4-dione
20. (z)-but-2-enedioic Acid;5-[[4-[2-[methyl(pyridin-2-yl)amino]ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione
21. 5-[[4-[2-[methyl(pyridin-2-yl)amino]ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione Maleate
22. Smr000471612
23. Avandia (tn)
24. Unii-kx2339dp44
25. 5-[4-[2-(n-methyl-n-(2-pyridyl)amino)ethoxy]benzyl]thiazolidine-2,4-dione Maleate
26. 5-[4-[2-[n-methyl-n-(2-pyridyl)amino)ethoxy]benzyl]thiazolidine-2,4-dione Maleate
27. Rosiglitazonemaleate
28. 2,4-thiazolidinedione, 5-[[4-[2-(methyl-2-pyridinylamino)ethoxy]phenyl]methyl]-, (2z)-2-butenedioate (1:1)
29. (+-)-5-(p-(2-(methyl-2-pyridylamino)ethoxy)benzyl)-2,4-thiazolidinedione Maleate (1:1)
30. 1217260-35-9
31. 5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)methyl]-1,3-thiazolidine-2,4-dione; But-2-enedioic Acid
32. Dsstox_cid_3569
33. Chembl843
34. Dsstox_rid_77086
35. Dsstox_gsid_23569
36. Schembl19023
37. Rosiglitazone Maleate- Bio-x
38. Mls001306472
39. Mls001401426
40. Dtxsid2023569
41. Rosiglitazone Maleate (jan/usp)
42. Hms2051b11
43. Hms2233i10
44. Hms3884h04
45. Rosiglitazone Maleate [mi]
46. Rosiglitazone Maleate [jan]
47. Tox21_302367
48. Ac-737
49. Mfcd03427306
50. Nsc717764
51. Rosiglitazone Maleate [vandf]
52. S2505
53. Rosiglitazone Maleate [mart.]
54. Akos015961534
55. Rosiglitazone Maleate [usp-rs]
56. Rosiglitazone Maleate [who-dd]
57. Ccg-100943
58. Ccg-208126
59. Cs-1692
60. Ks-5027
61. Nc00193
62. Nsc 717764
63. Ncgc00255233-01
64. 5-{[4-({2-[methyl(pyridin-2-yl)amino]ethyl}oxy)phenyl]methyl}-1,3-thiazolidine-2,4-dione (2z)-but-2-enedioate
65. Br164376
66. Hy-14600
67. Rosiglitazone Maleate [orange Book]
68. Cas-155141-29-0
69. Rosiglitazone Maleate [usp Monograph]
70. Sw197573-4
71. D00596
72. Rosiglitazone Maleate 100 Microg/ml In Methanol
73. 141r290
74. A809615
75. Q27888042
76. 2, 5-[[4-[2-(methyl-2-pyridinylamino) Ethoxy]phenyl]methyl]-, (2z)-2-butenedioate (1:1)
77. 5-[4-[2-[n-methyl-n-(2-pyridyl)amino]ethoxy]phenyl Methyl]thiazolidine-2,4-dione Maleate
78. 5-{4-[2-(methylpyridin-2-ylamino)ethoxy]benzyl}thiazolidine-2,4-dione Maleate
79. (+/-)-5-(p-(2-(methyl-2-pyridylamino)ethoxy)benzyl)-2,4-thiazolidinedione Maleate (1:1)
80. (z)-but-2-enedioate;2-[4-[(2,4-dioxo-1,3-thiazolidin-3-ium-5-yl)methyl]phenoxy]ethyl-methyl-pyridin-2-ylazanium
81. 5-((4-(2-(n-methyl-n-2-pyridylamino)ethoxy)phenyl)methyl)thiazolidine-2,4-dione Maleate
| Molecular Weight | 473.5 g/mol |
|---|---|
| Molecular Formula | C22H23N3O7S |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 9 |
| Exact Mass | 473.12567125 g/mol |
| Monoisotopic Mass | 473.12567125 g/mol |
| Topological Polar Surface Area | 171 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 588 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 6 | |
|---|---|
| Drug Name | Avandaryl |
| PubMed Health | Rosiglitazone (By mouth) |
| Drug Classes | Antidiabetic |
| Active Ingredient | rosiglitazone maleate; Glimepiride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 4mg; 2mg; 1mg; 8mg |
| Market Status | Prescription |
| Company | Sb Pharmco |
| 2 of 6 | |
|---|---|
| Drug Name | Avandia |
| Drug Label | AVANDIA (rosiglitazone maleate) is an oral antidiabetic agent which acts primarily by increasing insulin sensitivity. AVANDIA improves glycemic control while reducing circulating insulin levels. Rosiglitazone maleate is not chemically or functionally... |
| Active Ingredient | Rosiglitazone maleate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 4mg base; eq 2mg base; eq 8mg base |
| Market Status | Prescription |
| Company | Sb Pharmco |
| 3 of 6 | |
|---|---|
| Drug Name | Rosiglitazone maleate |
| PubMed Health | Rosiglitazone/Glimepiride (By mouth) |
| Drug Classes | Antidiabetic |
| Drug Label | AVANDIA (rosiglitazone maleate) is an oral antidiabetic agent which acts primarily by increasing insulin sensitivity. AVANDIA improves glycemic control while reducing circulating insulin levels. Rosiglitazone maleate is not chemically or functionally... |
| Active Ingredient | Rosiglitazone maleate |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | eq 4mg base; eq 2mg base; 8mg; 4mg; 2mg; eq 8mg base |
| Market Status | Tentative Approval; Prescription |
| Company | Mylan Pharma; Teva; Sandoz; Hikma Pharms; Roxane |
| 4 of 6 | |
|---|---|
| Drug Name | Avandaryl |
| PubMed Health | Rosiglitazone (By mouth) |
| Drug Classes | Antidiabetic |
| Active Ingredient | rosiglitazone maleate; Glimepiride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 4mg; 2mg; 1mg; 8mg |
| Market Status | Prescription |
| Company | Sb Pharmco |
| 5 of 6 | |
|---|---|
| Drug Name | Avandia |
| Drug Label | AVANDIA (rosiglitazone maleate) is an oral antidiabetic agent which acts primarily by increasing insulin sensitivity. AVANDIA improves glycemic control while reducing circulating insulin levels. Rosiglitazone maleate is not chemically or functionally... |
| Active Ingredient | Rosiglitazone maleate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 4mg base; eq 2mg base; eq 8mg base |
| Market Status | Prescription |
| Company | Sb Pharmco |
| 6 of 6 | |
|---|---|
| Drug Name | Rosiglitazone maleate |
| PubMed Health | Rosiglitazone/Glimepiride (By mouth) |
| Drug Classes | Antidiabetic |
| Drug Label | AVANDIA (rosiglitazone maleate) is an oral antidiabetic agent which acts primarily by increasing insulin sensitivity. AVANDIA improves glycemic control while reducing circulating insulin levels. Rosiglitazone maleate is not chemically or functionally... |
| Active Ingredient | Rosiglitazone maleate |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | eq 4mg base; eq 2mg base; 8mg; 4mg; 2mg; eq 8mg base |
| Market Status | Tentative Approval; Prescription |
| Company | Mylan Pharma; Teva; Sandoz; Hikma Pharms; Roxane |
AVANDAMET is indicated in the treatment of type 2 diabetes mellitus patients, particularly overweight patients:
- who are unable to achieve sufficient glycaemic control at their maximally tolerated dose of oral metformin alone.
- in triple oral therapy with sulphonylurea in patients with insufficient glycaemic control despite dual oral therapy with their maximally tolerated dose of metformin and a sulphonylurea (see section 4. 4).
Rosiglitazone is indicated in the treatment of type 2 diabetes mellitus:
as monotherapy
-in patients (particularly overweight patients) inadequately controlled by diet and exercise for whom metformin is inappropriate because of contraindications or intolerance
as dual oral therapy in combination with
-metformin, in patients (particularly overweight patients) with insufficient glycaemic control despite maximal tolerated dose of monotherapy with metformin
-a sulphonylurea, only in patients who show intolerance to metformin or for whom metformin is contraindicated, with insufficient glycaemic control despite monotherapy with a sulphonylurea
as triple oral therapy in combination with
-metformin and a sulphonylurea, in patients (particularly overweight patients) with insufficient glycaemic control despite dual oral therapy (see section 4. 4).
Rosiglitazone is indicated as oral monotherapy in type 2 diabetes mellitus patients, particularly overweight patients, inadequately controlled by diet and exercise for whom metformin is inappropriate because of contraindications or intolerance.
Rosiglitazone is also indicated for oral combination treatment in type 2 diabetes mellitus patients with insufficient glycaemic control despite maximal tolerated dose of oral monotherapy with either metformin or a sulphonylurea:
- in combination with metformin particularly in overweight patients.
- in combination with a sulphonylurea only in patients who show intolerance to metformin or for whom metformin is contraindicated.
Rosiglitazone is indicated as oral monotherapy in type 2 diabetes mellitus patients, particularly overweight patients, inadequately controlled by diet and exercise for whom metformin is inappropriate because of contraindications or intolerance.
Rosiglitazone is also indicated for oral combination treatment in type 2 diabetes mellitus patients with insufficient glycaemic control despite maximal tolerated dose of oral monotherapy with either metformin or a sulphonylurea:
- in combination with metformin particularly in overweight patients.
- in combination with a sulphonylurea only in patients who show intolerance to metformin or for whom metformin is contraindicated.
Alzheimer's Disease
Hypoglycemic Agents
Substances which lower blood glucose levels. (See all compounds classified as Hypoglycemic Agents.)
A10BD03
A10BG02
A10BG02
A10BG02

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
