
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
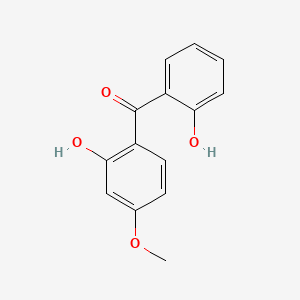

1. 131-53-3
2. 2,2'-dihydroxy-4-methoxybenzophenone
3. Benzophenone-8
4. Dioxybenzon
5. Advastab 47
6. Cyasorb Uv 24
7. Spectra-sorb Uv 24
8. (2-hydroxy-4-methoxyphenyl)(2-hydroxyphenyl)methanone
9. Methanone, (2-hydroxy-4-methoxyphenyl)(2-hydroxyphenyl)-
10. Cyasorb Uv 24 Light Absorber
11. Uf 2
12. Uv 24
13. (2-hydroxy-4-methoxyphenyl)-(2-hydroxyphenyl)methanone
14. Nsc-56769
15. Benzophenone, 2,2'-dihydroxy-4-methoxy-
16. Benzophenone-8;uv-24
17. Chebi:34208
18. B762xz551x
19. Nsc56769
20. 2-2'-dihydroxy-4-methoxybenzophenone
21. (2-hydroxy-4-methoxy-phenyl)-(2-hydroxyphenyl)methanone
22. 2,2'-dihydroxy-4-methoxy Benzophenone
23. 2,2'-dihydroxy-4-methoxy-benzophenone
24. Ncgc00016393-01
25. Dioxibenzonum
26. Cas-131-53-3
27. Dsstox_cid_2403
28. Dsstox_rid_76574
29. Dsstox_gsid_22403
30. Dioxibenzona
31. Dioxybenzonum
32. 2,2 Inverted Exclamation Marka-dihydroxy-4-methoxybenzophenone
33. Dioxybenzonum [inn-latin]
34. Dioxibenzona [inn-spanish]
35. Ccris 6231
36. Sr-05000001610
37. Einecs 205-026-8
38. Nsc 56769
39. Brn 2055461
40. Unii-b762xz551x
41. Ai3-25363
42. Dioxybenzone [usan:usp:inn]
43. Uv-24
44. 2-(2-hydroxybenzoyl)-5-methoxyphenol
45. Spectro-sorb Uv 24
46. Spectra-sorb Uv-24
47. Spectrum_000978
48. Prestwick0_000898
49. Prestwick1_000898
50. Prestwick2_000898
51. Prestwick3_000898
52. Spectrum2_001032
53. Spectrum3_000399
54. Spectrum4_000519
55. Spectrum5_000913
56. Dioxybenzone (usp/inn)
57. Dioxybenzone [mi]
58. Dioxybenzone [inn]
59. Dioxybenzone [usan]
60. Dioxybenzone [vandf]
61. Schembl15894
62. Bspbio_000716
63. Bspbio_002217
64. Dioxybenzone [mart.]
65. Kbiogr_001097
66. Kbioss_001458
67. Mls002154056
68. Bidd:er0352
69. Dioxybenzone [usp-rs]
70. Dioxybenzone [who-dd]
71. Spectrum1500255
72. Spbio_001243
73. Spbio_002925
74. Benzophenone-8 [inci]
75. Bpbio1_000788
76. Chembl1326877
77. Dtxsid3022403
78. Dioxybenzone, Analytical Standard
79. Hsdb 8474
80. Kbio2_001458
81. Kbio2_004026
82. Kbio2_006594
83. Kbio3_001437
84. Zinc37293
85. Component Of Solaquin (salt/mix)
86. Am720
87. Hms1570d18
88. Hms1920i04
89. Hms2091o12
90. Hms2097d18
91. Hms2235j06
92. Hms3371g16
93. Hms3714d18
94. Pharmakon1600-01500255
95. 2,2'-dihydroxy-methoxybenzophenone
96. Hy-b0966
97. Dioxybenzone [usp Monograph]
98. Tox21_110417
99. Tox21_200993
100. Ccg-40196
101. Mfcd00002218
102. Nsc756742
103. S4607
104. Akos015856203
105. Benzophenone,2'-dihydroxy-4-methoxy-
106. Tox21_110417_1
107. 2,2'-dihydroxy-4-methoxybenzo-phenone
108. 2,2\'-dihydroxy-4-methoxybenzophenone
109. Cs-4451
110. Db11221
111. Ks-5318
112. Nsc-756742
113. Ncgc00016393-02
114. Ncgc00016393-03
115. Ncgc00016393-04
116. Ncgc00016393-07
117. Ncgc00094657-01
118. Ncgc00094657-02
119. Ncgc00258546-01
120. Smr001233373
121. Sbi-0051353.p003
122. 2,2'-dihydroxy-4-methoxybenzophenone, 98%
123. Ab00051973
124. D0586
125. Ft-0609243
126. D03853
127. D89684
128. Ab00051973_08
129. A806277
130. J-506801
131. Q2533641
132. Sr-05000001610-1
133. Sr-05000001610-3
134. W-108331
135. Brd-k22193694-001-05-2
136. Brd-k22193694-001-08-6
137. S4607 2,2 Inverted Exclamation Marka-dihydroxy-4-methoxyb
138. Dioxybenzone, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 244.24 g/mol |
|---|---|
| Molecular Formula | C14H12O4 |
| XLogP3 | 3.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 244.07355886 g/mol |
| Monoisotopic Mass | 244.07355886 g/mol |
| Topological Polar Surface Area | 66.8 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 292 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPL THER/ BACKGROUND: Sunscreen compounds with added benefit of skin cancer prevention have both public and commercial interests. Our earlier study using the Epstein-Barr virus early antigen in vitro assay reported on skin cancer chemoprevention potential of benzophenone sunscreens. We now report the in vivo antitumor activity of two of the benzophenone sunscreens which tested positively in the in vitro assay, octabenzone (UV-1) and dioxybenzone (UV-2), in the two-stage mouse skin carcinogenesis model using (+/-)-(E)-4-methyl-2-[-(E)-hydroxyamino]-5-nitro-6-methoxy-3-hexanamide (NOR-1) as inducer and 12-O-tetradecanoyl-phorbol-13-acetate (TPA) as promoter. MATERIALS AND METHODS: Pathogen-free, female hairless mice of HOS:HR-1 strain, 15 animals per control and test groups, were used. Skin tumors were induced by a single dose of NOR-1 (390 nmol in 100 uL of acetone). One week later, TPA (1.7 nmol in 100 uL of acetone) was applied to skin twice weekly for 20 weeks as tumor a promoter. The test compounds UV-I or UV-2 were administered at 0.0025% to mice through drinking water ad libitum, starting one week prior to and stopping one week after tumor initiation. All animals were examined weekly for the development of skin papillomas. RESULTS: In both UV-1- and UV-2-treated mice, a two-week delay in tumor appearance, and significant inhibition (p<0.001) of tumor incidence (50% and 60%, respectively) and tumor burden (papilloma inhibition/mouse, 50% and 70%, respectively) were observed when compared to the positive control group. UV-2 (dihydroxy derivative) was a more potent inhibitor of skin tumor than UV-1 (monohydroxy derivative), which followed their antioxidant activity ranking. CONCLUSION: The results affirm the skin cancer chemoprevention potential of orally-ingested benzophenone sunscreens in mice and warrant studies in humans to validate synergistic protection achievable by complementation of oral and topical sunscreen usage.
PMID:23749905 Rao GS et al; Anticancer Res 33 (6): 2535-40 (2013)
Sunscreen agents are used to prevent sunburn and premature aging of the skin, and to reduce the incidence of solar or actinic-induced keratoses, skin cancers, tanning, and other harmful effects of the sun. Some data suggest that carcinogenesis and photoaging can occur at doses of UV radiation below that required to produce a sunburn (i.e., suberythemal doses). Most clinicians agree that the liberal and regular use of an effective sunscreen is therapeutically desirable and not just cosmetically desirable, especially in light-skinned people with blue eyes, red hair, and/or freckles who are most susceptible to the acute and chronic harmful effects of sunlight. /Sunscreens/
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018
Because the absorptive characteristics of skin of children younger than 6 months of age may differ from those of adults and because the immaturity of metabolic and excretory pathways of these children may limit their ability to eliminate any percutaneously absorbed sunscreen agent, sunscreen products should be used in children younger than 6 months of age only as directed by a clinician. It is possible that the characteristics of geriatric skin also differ from those of skin in younger adults, but these characteristics and the need for special considerations regarding use of sunscreen preparations in this age group are poorly understood. /Sunscreens/
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018
If skin irritation or a rash occurs during use of a sunscreen product, use of the sunscreen should be discontinued and the sunscreen washed off. If irritation persists, a physician should be consulted. Contact of sunscreen agents with the eyes should be avoided. If the sunscreen comes in contact with the eyes, the affected eye(s) should be flushed thoroughly with water. /Sunscreens/
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018
Little information is available regarding the safety of chronic sunscreen usage, but commercially available physical and chemical sunscreens appear to have a low incidence of adverse effects. Derivatives of PABA, benzophenone, cinnamic acid, and salicylate and 2-phenylbenzimidazole-5-sulfonic acid have caused skin irritation including burning, stinging, pruritus, and erythema on rare occasions. Skin irritation produced by padimate A appears to be dose related. /Sunscreens/
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018
Although it has been suggested that benzophenone derivatives may protect against photosensitivity reactions to photosensitizing drugs (e.g., chlordiazepoxide, chlorpromazine, demeclocycline, hydrochlorothiazide, nalidixic acid, nystatin, sulfisoxazole), most clinicians agree that these sunscreens provide, at most, only limited protection for patients who are sensitive to these drugs. /Benzophenone derivatives/
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018
Even when using a sunscreen, prolonged sunlight exposure should be avoided and protective clothing should be worn by all persons, particularly those that are fair-skinned, blue-eyed, or blond. Until a protective tan develops, initial sunlight exposures should be limited to short periods, which may be gradually lengthened. /Sunscreens/
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018
Indicated for use as an active sunscreen agent.
Dioxybenzone is a sunscreen agent and chemical UV filter that absorbs UV-B rays and UV-AII rays to limit their penetration into human skin. In a screening protocol consisting of the _in vitro_ EBV-EA activation assay followed by the _in vivo_ confirmation test in the two-stage mouse skin cancer model utilizing NOR-1 as inducer and TPA as promoter of tumour, dioxybenzone exhibited a significant chemopreventive activity against mouse skin carcinogenesis which correlated with their antioxidant potency. There is some evidence that suggests some benzophenones and their hydroxylated metabolites act as weak estrogens in the environment; however similar effect of dioxybenzone has not been established.
Absorption
Dioxybenzone is a derivative of benzophenone. In monkeys, percutaneous absorption of benzophenone was observed. Other derivatives of benzophenone are capable of crossing the skin via direct penetration through the intercellular laminae of the stratum corneum (SC) or by passive diffusion by high-concentration gradient into the systemic circulation, where they are transported to different tissues including liver and brain.
Route of Elimination
No pharmacokinetic data available.
Volume of Distribution
No pharmacokinetic data available.
Clearance
No pharmacokinetic data available.
Information on the cutaneous absorption, distribution, and elimination of most topically applied sunscreen agents is limited. Solvents used in sunscreen products affect the stability and binding of the drug to the skin; in general, alcoholic solvents allow for the most rapid and deepest epidermal penetration of sunscreens. It appears that sunscreen agents are absorbed by the intact epidermis to varying degrees. /Sunscreens/
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018
No pharmacokinetic data available.
The pharmacokinetics of benzophenone-3 (BZ-3) was studied in rats. Male Sprague-Dawley-rats were administered 0 or 100 mg/kg oxybenzone orally. Blood samples were collected from some rats for up to 20 hours post dosing and analyzed for plasma oxybenzone by high performance liquid chromatography. Urine, feces, and expired air samples were collected for up to 96 hours and analyzed for BZ-3 metabolites. Selected rats were killed 6 hours after dosing to determine the tissue distribution of BZ-3. The kinetic behavior of oxybenzone was investigated by applying the plasma BZ-3 data to standard pharmacokinetic models. The pharmacokinetic behavior of BZ-3 in the blood could be described by a two compartment model, the halflives for distribution and elimination being 0.88 and 15.90 hours, respectively. The absorption halflife was 0.71 hour. The maximum plasma concentration, 25.6 micrograms per milliliter, occurred 3 hours after dosing. The liver had the largest total concentration of BZ-3, 6.47% of the dose, followed by the kidney, spleen, intestines, and heart in that order. BZ-3 was detected in the testes only after acid hydrolysis and in only one of six rats; however, the concentration represented 1.8% of the dose. Approximately 60% of the dose was excreted in the urine and feces over 96 hours. Urine was the predominant route of excretion. Most of the excreted dose consisted of compounds conjugated with macromolecules. Enzyme hydrolysis of the urine samples with beta-glucuronidase showed that most of the excreted dose was conjugated with glucuronic-acid. Identified metabolites included 2,4-dihydroxybenzophenone, 2,2'-dihydroxy-4-methoxybenzophenone, and 2,3,4-trihydroxybenzophenone. The authors conclude that following oral administration, oxybenzone is rapidly absorbed from the gastrointestinal tract and distributed primarily to the liver, kidneys, and testes, indicating that the liver may be the major organ involved in BZ-3 elimination.
Kadry AM et al; Journal of Applied Toxicology 15 (2): 97-102 (1995)
Metabolism of the ultraviolet absorber benzophenone-3 (BZ3) by Sprague-Dawley-rats was studied. Rats were fed 100 mg/kg BZ3 by gavage and blood, tissue, urine, and fecal samples were examined at various time points. BZ3 and its metabolites were identified in plasma as soon as 5 minutes after administration. 2,4-Dihydroxybenzophenone (DHB), 2,2'-dihydroxy-4-methoxybenzophenone, and 2,3,4-trihydroxybenzophenone were detected in the blood after 30 minutes. DHB was the major metabolite found in tissue, urine, and fecal samples. The parent compound and metabolites appeared bound to macromolecules or in conjugated forms in plasma, as free compounds and conjugates in tissues, and extensively conjugated in feces and urine. The primary route of elimination was urinary and O-dealkylation was found to be the major metabolic pathway.
Okereke CS et al; Drug Metabolism and Disposition 21 (5): 788-791 (1993)
The metabolism and fate of benzophenone-3 (BZ-3) was studied in male Sprague-Dawley-rats and male B6C3F1-mice after oral administration of 100mg/kg body weight. Blood samples were collected at 5 minutes to 20 hours after administration. For a tissue distribution study, tissue samples were obtained 6 hours after administration of the BZ-3. For urinary and fecal excretion studies, mice and rats were placed in glass metabolism cages for 96 hours after administration of BZ-3. In rats, BZ-3 exhibited a biphasic elimination in plasma with alpha and beta elimination half lives of 0.9 and 15.9 hours, as compared to a single phase elimination half life of 1.8 hours in mice. Absorption was faster and peak plasma concentrations were reached faster in mice than rats. In the tissues studied, accumulation of the parent compound was highest in the liver, with higher amounts in the rat than mouse. 2,4-Dihydroxybenzophenone (DHB) was the major metabolite in the tissues, with higher amounts detected in the rat than the mouse. In rats, the urine was the major route of excretion of BZ-3 and DHB. In mice, elimination was divided between the urine and feces, with 2,3,4-trihydroxybenzophenone (THB) as the primary metabolite. Trace amounts of 2,2'-dihydroxy-4-methoxybenzophenone (DHMB) were found in the urine and feces of both species. The peak excretion in the urine of both the parent compound and DHB was earlier in rats than in mice. Most of the excretion of the BZ-3 and DHB in the feces was complete within 24 hours for both species, although the total fecal excretion for the parent compound was almost double in mice than rats, and for DHB was significantly less in mice than rats. The authors postulate that variations in the absorption rates, distribution patterns, and metabolism of BZ-3 in rats and mice may be related to species specific quantitative and qualitative differences in enzyme activities.
Okereke CS, Abdel-Rahman MS; Toxic Substances Journal 13 (4): 239-251 (1994)
No pharmacokinetic data available.
Emitted by the sun, UVA-II rays, which range at 320400 nm and are not absorbed by the ozone layer, and UVB rays, which range 290320 nm and are partially absorbed by the ozone layer and exert a damaging effect on human skin, including basal cell carcinoma and melanoma. As a chemical filter, dioxybenzone absorb these rays to prevent their penetration into the skin and attenuate long-term skin damage caused by UV radiation from the sun. In a rat uterine cytosolic estrogen receptor (ER) competitive binding assay, dioxybenzone was not found to be a ER-binder.
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?