

Synopsis
Synopsis
0
CEP/COS
0
EU WC
0
VMF
0
Listed Suppliers
0
FDF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
0
EDQM
0
USP
0
JP
0
Others
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
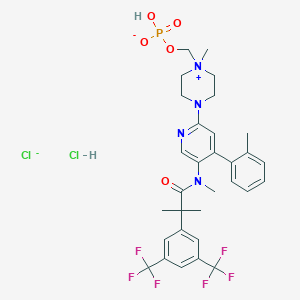

| Molecular Weight | 760.5 g/mol |
|---|---|
| Molecular Formula | C31H36Cl2F6N4O5P- |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 8 |
| Exact Mass | 759.1704566 g/mol |
| Monoisotopic Mass | 759.1704566 g/mol |
| Topological Polar Surface Area | 106 A^2 |
| Heavy Atom Count | 49 |
| Formal Charge | -1 |
| Complexity | 1100 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
| 1 of 1 | |
|---|---|
| Drug Name | AKYNZEO |
| Active Ingredient | FOSNETUPITANT CHLORIDE HYDROCHLORIDE; PALONOSETRON HYDROCHLORIDE |
| Company | HELSINN HLTHCARE (Application Number: N210493) |
DRUG PRODUCT COMPOSITIONS

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
We have 2 companies offering 08-PNET
Get in contact with the supplier of your choice:


LOOKING FOR A SUPPLIER?
