

Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
FDF
0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
0
EDQM
0
USP
0
JP
0
Others
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
Finished Drug Prices
NA
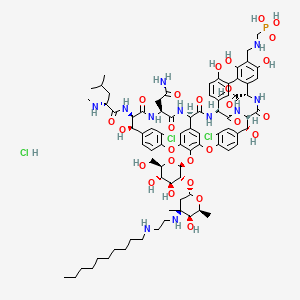

1. Td 6424
2. Td-6424
3. Td6424
4. Telavancin
5. Vibativ
1. Vibativ
2. Telavancin Hcl
3. 560130-42-9
4. Td-6424
5. Td 6424
6. Telavancin Hydrochloride [usan]
7. Televancin Hydrochloride
8. 0701472zg0
9. Telavancin Hydrochloride (usan)
10. Unii-0701472zg0
11. Vibativ (tn)
12. Chembl3301680
13. Chebi:71226
14. Telavancin Hydrochloride [mi]
15. Telavancin Hydrochloride [mart.]
16. Telavancin Hydrochloride [vandf]
17. Televancin Hydrochloride [vandf]
18. Telavancin Hydrochloride [who-dd]
19. Telavancin Hydrochloride [orange Book]
20. D06057
21. Q47495786
22. 380636-75-9
23. Vancomycin, N(sup 3'')-(2-(decylamino)ethyl)-29-(((phosphonomethyl)amino)methyl)-, Monohydrochloride
24. Vancomycin, N3'-(2-(decylamino)ethyl)-29-(((phosphonomethyl)amino)methyl)-, Monohydrochloride
| Molecular Weight | 1792.1 g/mol |
|---|---|
| Molecular Formula | C80H107Cl3N11O27P |
| Hydrogen Bond Donor Count | 24 |
| Hydrogen Bond Acceptor Count | 31 |
| Rotatable Bond Count | 30 |
| Exact Mass | 1789.614107 g/mol |
| Monoisotopic Mass | 1789.614107 g/mol |
| Topological Polar Surface Area | 598 Ų |
| Heavy Atom Count | 122 |
| Formal Charge | 0 |
| Complexity | 3490 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 18 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Vibativ |
| PubMed Health | Telavancin (Injection) |
| Drug Classes | Antibiotic |
| Drug Label | VIBATIV contains telavancin hydrochloride, a lipoglycopeptide antibacterial that is a synthetic derivative of vancomycin. The chemical name of telavancin hydrochloride is vancomycin,N3''-[2-(decylamino)ethyl]-29-[[(phosphono-methyl)-amino]-methy |
| Active Ingredient | Telavancin hydrochloride; Telavancin |
| Dosage Form | Powder; Injectable |
| Route | injection; Iv (infusion) |
| Strength | eq 750mg base/vial; 250mg; 750mg; eq 250mg base/vial |
| Market Status | Prescription |
| Company | Theravance; Theravance Biopharma |
| 2 of 2 | |
|---|---|
| Drug Name | Vibativ |
| PubMed Health | Telavancin (Injection) |
| Drug Classes | Antibiotic |
| Drug Label | VIBATIV contains telavancin hydrochloride, a lipoglycopeptide antibacterial that is a synthetic derivative of vancomycin. The chemical name of telavancin hydrochloride is vancomycin,N3''-[2-(decylamino)ethyl]-29-[[(phosphono-methyl)-amino]-methy |
| Active Ingredient | Telavancin hydrochloride; Telavancin |
| Dosage Form | Powder; Injectable |
| Route | injection; Iv (infusion) |
| Strength | eq 750mg base/vial; 250mg; 750mg; eq 250mg base/vial |
| Market Status | Prescription |
| Company | Theravance; Theravance Biopharma |
Vibativ is indicated for the treatment of adults with nosocomial pneumonia including ventilator-associated pneumonia, known or suspected to be caused by methicillin-resistant Staphylococcus aureus (MRSA).
Vibativ should be used only in situations where it is known or suspected that other alternatives are not suitable.
Consideration should be given to official guidance on the appropriate use of antibacterial agents.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J01XA03
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
