


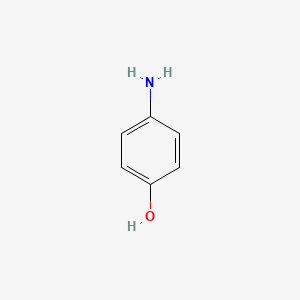

1. 4-aminophenol Conjugate Monoacid
2. 4-aminophenol Hydrochloride
3. 4-aminophenol Monopotassium Salt
4. 4-aminophenol Monosodium Salt
5. 4-aminophenol Sulfate
6. 4-aminophenol Sulfate (2:1)
7. 4-aminophenol, 18o-labeled
8. 4-aminophenol, 3h-labeled
9. 4-aminophenol, Ion(1+)
10. 4-hydroxyaniline
11. P-aminophenol
12. P-aminophenol Phosphate
13. Para-aminophenol
1. 123-30-8
2. P-aminophenol
3. 4-hydroxyaniline
4. P-hydroxyaniline
5. Phenol, 4-amino-
6. Paranol
7. 4-aminobenzenol
8. Certinal
9. Activol
10. Citol
11. Azol
12. P-hydroxyphenylamine
13. Fouramine P
14. Ursol P Base
15. Rodinal
16. Phenol, P-amino-
17. Benzofur P
18. Fourrine P Base
19. Pelagol P Base
20. Tertral P Base
21. Ursol P
22. 4-amino-1-hydroxybenzene
23. Durafur Brown Rb
24. Furro P Base
25. Nako Brown R
26. Renal Ac
27. Fourrine 84
28. Zoba Brown P Base
29. 4-amino-phenol
30. Para-aminophenol
31. Unal
32. 1-amino-4-hydroxybenzene
33. Basf Ursol P Base
34. C.i. Oxidation Base 6
35. Pelagol Grey P Base
36. P-aminofenol
37. P-aminofenol [czech]
38. Aminophenol, P-
39. Nsc 1545
40. C.i. 76550
41. Paramidophenol
42. P-aminobenzenol
43. 4-amino Phenol
44. 4-hydroxybenzenamine
45. 4-hydroxyphenylamine
46. Ci 76550
47. Chebi:17602
48. Mfcd00007869
49. C.i. Oxidation Base 6a
50. R7p8frp05v
51. Phenol, 4-amino-, Homopolymer
52. Dtxsid3024499
53. Nsc-1545
54. P-aminophenol [un2512] [poison]
55. Ncgc00090816-01
56. Ncgc00090816-02
57. Dsstox_cid_4499
58. Dsstox_rid_77429
59. Dsstox_gsid_24499
60. 25668-00-2
61. 4nl
62. Cas-123-30-8
63. Ccris 4146
64. Hsdb 2640
65. Einecs 204-616-2
66. Unii-r7p8frp05v
67. Paraaminophenol
68. Energol
69. Kodelon
70. Takatol
71. Para Aminophenol
72. Ai3-14872
73. P-amino-phenol
74. 4-aminophenol B
75. Para-amino-phenol
76. 4-hydroxy-aniline
77. Para-hydroxyaniline
78. Para Amino Phenol
79. 4-azaniumylphenolate
80. Mesalamine Impurity A
81. Phenol Derivative, 9
82. (raloxifene Impurity)
83. Furro P (salt/mix)
84. Peltol P (salt/mix)
85. Pelagol Cp (salt/mix)
86. Futramine P (salt/mix)
87. Wln: Zr Dq
88. 4-aminophenol-[13c6]
89. Bmse000462
90. Epitope Id:117708
91. Ec 204-616-2
92. 4-aminophenol, >=98%
93. P-aminophenol [mi]
94. Schembl3424
95. Chembl1142
96. P-aminophenol [inci]
97. Durafur Brown R (salt/mix)
98. Mls001066356
99. 4-aminophenol [hsdb]
100. Pelagol Grey Cp (salt/mix)
101. Sgcut00256
102. 4-aminophenol [usp-rs]
103. Schembl15663694
104. Bdbm26195
105. Nsc1545
106. Bcp25857
107. To_000006
108. Zinc4623758
109. 4-aminophenol, >=99% (hplc)
110. Tox21_113242
111. Tox21_113477
112. Tox21_201030
113. Stk286017
114. 4-aminophenol [usp Impurity]
115. 4-aminophenol, Technical Grade, 95%
116. Akos000119829
117. Akos016371265
118. Tox21_113477_1
119. Am86423
120. Ccg-266045
121. Db14144
122. Sb40549
123. Un 2512
124. Ncgc00090816-03
125. Ncgc00090816-04
126. Ncgc00090816-05
127. Ncgc00258583-01
128. As-54109
129. Smr000471841
130. Mesalazine Impurity A [ep Impurity]
131. A0384
132. Cs-0006652
133. Ft-0617593
134. Paracetamol Impurity K [ep Impurity]
135. C02372
136. P17510
137. 4-aminophenol, Pestanal(r), Analytical Standard
138. Aj-333/25022099
139. J-004908
140. J-514454
141. Q2548040
142. Z57127517
143. F2190-0438
144. 4-aminophenol, United States Pharmacopeia (usp) Reference Standard
145. Mesalazine Impurity A, European Pharmacopoeia (ep) Reference Standard
146. 4-aminophenol, 250 Mug/ml In Acetonitrile, Certified Reference Material, Ampule Of 1 Ml
147. 1-amino-4-hydroxybenzene 4-amino-1-hydroxybnzene 4-aminobenzenol 4-amino-pheno
148. 4-aminophenol (acetaminophen Related Compound K) (paracetamol Impurity K), Pharmaceutical Secondary Standard; Certified Reference Material
149. 4-aminophenol; P-aminophenol; 1-amino-4-hydroxybenzene; 4-amino-1-hydroxybenzene; 4-hydroxyaniline
| Molecular Weight | 109.13 g/mol |
|---|---|
| Molecular Formula | C6H7NO |
| XLogP3 | 0 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 109.052763847 g/mol |
| Monoisotopic Mass | 109.052763847 g/mol |
| Topological Polar Surface Area | 46.2 Ų |
| Heavy Atom Count | 8 |
| Formal Charge | 0 |
| Complexity | 66.9 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
4.4 = very toxic: probable oral lethal dose (human) 50-500 mg/kg, between 1 teaspoon & 1 oz for 70 kg person (150 lb). ... causes methemoglobinemia, but less toxic than aniline in animals. ... diln used in photography do not have significant percutaneous toxicity in most cases.
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-141
Mutagens
Chemical agents that increase the rate of genetic mutation by interfering with the function of nucleic acids. A clastogen is a specific mutagen that causes breaks in chromosomes. (See all compounds classified as Mutagens.)
An absorption and excretion study of permanent hair dye intermediates containing 14-C was conducted in hairless Wistar rats under conditions of oxidation hair dyeing (ie, intermediates were mixed with H202 immediately before application). The cutaneous penetration of 14C-4-aminophenol (PAP) alone and in admixture with a nonradioactive coupler (3-amino-6-methylphenol, a 3-aminophenol (MAP) derivative) and that of the resultant 14C-indamine (N-[4-hydroxyphenyl}-3-amino-6-methyl benzoquinone imine) was determined. Hair dye solutions containing uniformly labeled PAP (0.75% or 70 nM) in a simple vehicle were applied to a 10 sq cm dorsal surface of up to 5 rats. Doses of PAP of 0.14 uM/sq cm, 0.69 uM/sq cm, and 3.44/ uM/sq cm yielded respective concentrations of 15.9 +/- 4.76 nM/sq cm, 52.04 +/- 6.73 nM/sq cm, and 58.4 +/- 11.5 nM/sq cm, which penetrated the skin and were detected in the excreta, the viscera, and the skin (excluding PAP found at the site of application) of the treated rats after 4 days. At the highest 14C-PAP concentration applied, the total quantity of 14C-PAP detected per sq cm of skin was approximately the same for PAP alone as for PAP mixed with nonradioactive MAP coupler (56.8 +/- 4.0 nM/sq cm). Penetration of the 14C-indamine was approximately 17 times less (3.6 +/- 0.46 nM) than that of PAP or of the mixture of PAP with the nonradioactive coupler..
Cosmetic Ingredient Expert Review Panel; Final Report on the Safety Assessment of p-Aminophenol, m-Aminophenol, and o-Aminophenol; Journal of the American College of Toxicology 7 (3): 279-333 (1988).
... Hepatocytes prepared from male Sprague-Dawley rats ... converted para-aminophenol (PAP) to two major metabolites (PAP-GSH conjugates and PAP-N-acetylcysteine conjugates) and several minor metabolites [PAP-O-glucuronide, acetaminophen (APAP), APAP-O-glucuronide, APAP-GSH conjugates, and 4-hydroxyformanilide]. Preincubating hepatoyctes with 1-aminobenzotriazole, an inhibitor of cytochromes P450, did not alter the pattern of PAP metabolism. In conclusion, we found that PAP was metabolized in hepatocytes predominantly to PAP-GSH conjugates and PAP-N-acetylcysteine conjugates in sufficient quantities to account for the nephrotoxicity of PAP.
PMID:10901695 Yan Z et al; Drug Metab Dispos 28 (8): 880-6 (2000)
... The hepatic metabolism of p-aminophenol in Wistar rats and the cytotoxicity of formed glutathione S-conjugates in rat renal epithelial cells /were examined/. After ip application of p-aminophenol (100 mg/kg), the following metabolites were identified in rat bile: 4-amino-2-(glutathion-S-yl)phenol, 4-amino-3-(glutathion-S-yl)-phenol, 4-amino-2,5-bis(glutathion-S-yl)phenol, 4-amino-2,3,5(or 6)-tris(glutathion-S-yl)phenol, an aminophenol conjugate (likely a sulfate or glucuronide), acetaminophen glucuronide, and 3-(glutathion-S-yl)acetaminophen. 4-Amino-3-(glutathion-S-yl)phenol, 4-amino-2,5-bis(glutathion-S-yl)phenol, and 4-amino-2,3,5(or 6)-tris(glutathion-S-yl)phenol induced a dose- and time-dependent loss of cell viability in rat kidney cortical cells. Cell killing was significantly reduced by inhibition of gamma-glutamyl transpeptidase with Acivicin. p-Aminophenol was also toxic to renal epithelial cells. Coincubation of p-aminophenol with tetraethylammonium bromide, a competitive inhibitor of the organic cation transporter, and with SKF-525A, an inhibitor of cytochrome P450, protected cells from p-aminophenol-induced toxicity. p-Aminophenol would thus be accumulated in the kidney mainly by organic cation transport systems, which are concentrated in the S-1 segment of the proximal tubule. However, p-aminophenol toxicity in vivo is directed toward the S-2 and S-3 segments, which are rich in gamma-glutamyl transpeptidase. These results and the observation that biliary cannulation and glutathione depletion reduce p-aminophenol nephrotoxicity suggest that the biosynthesis of toxic glutathione conjugates is responsible for p-aminophenol nephrotoxicity in vivo. The aminophenol glutathione S-conjugates formed induce p-aminophenol nephrotoxicity by a pathway dependent on gamma-glutamyl transpeptidase.
PMID:1631900 Klos C et al; Toxicol Appl Pharmacol 115 (1): 98-106 (1992)
4-Aminophenol in the presence of oxyhemoglobin forms numerous adducts with glutathione (GSH). Using (14)C-4-aminophenol and (3)H-glutathione, ten different thioethers were isolated, by HPLC, with isotope ratios of 1:1, 1:2, 1:3, respectively ... In erythrocytes of humans and dogs, and in dog blood, in vivo, the same pattern of 4-aminophenol conjugates with GSH was found. In vivo, 5% of administered 4-aminophenol is converted into thioethers within erythrocytes, accompanied by a 60% decrease in the cellular GSH, indicating the role of erythrocytes in the biotransformation of xenobiotics.
PMID:3245228 Eckert KG; Xenobiotica 18 (11): 1319-26 (1988)
p-Aminophenol yields p-acetamidophenol, p-aminophenyl-beta-d-glucuronide, p-aminophenyl sulfate, 4-aminoresorcinol, and p-methylaminophenol in rabbit.
European Commission, ESIS; IUCLID Dataset, 4-Aminophenol (123-30-8) p.15 (2000 CD-ROM edition). Available from, as of May 17, 2010: https://esis.jrc.ec.europa.eu/
For more Metabolism/Metabolites (Complete) data for 4-Aminophenol (12 total), please visit the HSDB record page.
4-aminophenol is a known human metabolite of aniline.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
... Markers of ER stress (XBP1 messenger RNA processing and protein expression; GRP78 and GRP94 upregulation) and ER-mediated cell death (caspase-12 and calpain activation) were examined in kidney tissue of rats exposed to nephrotoxic doses of cisplatin (CIS), gentamicin (GEN), and p-aminophenol (PAP), a nephrotoxic metabolite of acetaminophen. XBP1 signaling was observed with all three drugs and was associated with increased expression of GRP94 and GRP78 in GEN- and PAP-treated animals, but not after CIS exposure. m-Calpain expression was increased after 7 days of CIS treatment, whereas it was decreased in PAP-treated rats. Caspase-12 cleavage products were increased after CIS, GEN, and PAP administration. The results of this study demonstrate that three clinically relevant nephrotoxic drugs are all associated with changes in markers of ER stress and ER-mediated cell death in vivo ...
PMID:17567590 Peyrou M et al; Toxicol Sci 99 (1): 346-53 (2007)
4-Aminophenol (4-AP), D-serine, and cisplatin are established rodent nephrotoxins that damage proximal tubules within the renal cortex ... High throughput 2D gel proteomics to profile protein changes in the plasma of compound-treated animals, ... /demonstrated/ several markers of kidney toxicity. Male F344 and Alpk rats were treated with increasing doses of 4-AP, D-serine, or cisplatin, and plasma samples were collected over time ... Several isoforms of the rat-specific T-kininogen protein were identified in each study. T-kininogen was elevated in the plasma of 4-AP-, D-serine-, and cisplatin-treated animals at early time points, returning to baseline levels 3 weeks after treatment. The protein was not elevated in the plasma of control animals or those treated with nontoxic compounds. /It was proposed/ that T-kininogen may be required to counteract apoptosis in proximal tubular cells in order to minimize tissue damage following a toxic insult. In addition, T-kininogen may be required to stimulate localized inflammation to aid tissue repair ... Several isoforms of the inter-alpha inhibitor H4P heavy chain /were identified/ in the 4-AP and D-serine studies. In each case, the protein expression levels in the blood samples paralleled the extent of kidney toxicity, highlighting the correlation between protein alterations and clinical chemistry endpoints. A further set of proteins correlating with kidney damage was found to be a component of the complement cascade and other blood clotting factors, indicating a contribution of the immune system to the observed toxicity. These observations underscore the value of proteomics in identifying new biomarkers and in the elucidation of mechanisms of toxicity.
PMID:12657746 Bandara LR et al; Toxicol Sci 73 (1): 195-206 (2003)
One of the primary effects found from exposure to p-aminophenol is the formation of methemoglobin. This effect has been found in many species with a wide degree of susceptibility. The oxidation of hemoglobin to methemoglobin interferes with normal oxygen transport functions of hemoglobin and can result in a chemical asphyxia (usually at levels of 60% or more). It is believed that p-aminophenol forms a covalent bond with the reactive -SH groups of hemoglobin and transfers electrons to oxygen to created methemoglobin.
European Commission, ESIS; IUCLID Dataset, 4-Aminophenol (123-30-8) p.43 (2000 CD-ROM edition). Available from, as of May 17, 2010: https://esis.jrc.ec.europa.eu/
p-Aminophenol is a significantly toxic chemical and one mechanism associated with its cytotoxicity has been attributed to its activity as a tissue respiratory (oxidative phosphorylation) inhibitor ... .
Clayton, G.D., F.E. Clayton (eds.) Patty's Industrial Hygiene and Toxicology. Volumes 2A, 2B, 2C, 2D, 2E, 2F: Toxicology. 4th ed. New York, NY: John Wiley & Sons Inc., 1993-1994., p. 973
Reply 
Reply 
Reply 

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] 

