




1. 31251-41-9
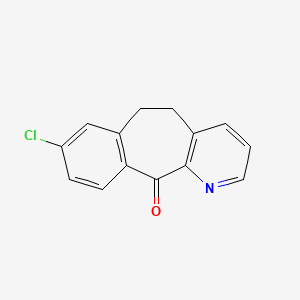
2. 8-chloro-5h-benzo[5,6]cyclohepta[1,2-b]pyridin-11(6h)-one
3. 8-chloro-5,6-dihydro-11h-benzo(5,6)cyclohepta(1,2-b)pyridin-11-one
4. Loratadine Related Compound C
5. Loratadine Ep Impurity B
6. 8-chloro-10,11-dihydro-4-aza-5h-dibenzo-(a,d)cycloheptan-5-one
7. 8-chloro-10,11-dihydro-4-aza-5h-dibenzo[a,d]cycloheptan-5-one
8. 11h-benzo[5,6]cyclohepta[1,2-b]pyridin-11-one, 8-chloro-5,6-dihydro-
9. 13-chloro-4-azatricyclo[9.4.0.03,8]pentadeca-1(11),3(8),4,6,12,14-hexaen-2-one
10. 8-chloro-10,11-dihydro-4-aza-5h-benzo[a,d] Cycloheptan-5-one
11. Y8ma5g8500
12. Mfcd00800222
13. 8-chloro-5,6-dihydro-11h-benzo[5.6]cyclohepta[1,2-b]pyridin-11-one
14. Unii-y8ma5g8500
15. 8-chloro-5,6-dihydrobenzo[1,2]cyclohepta[2,4-b]pyridin-11-one
16. Loratadine Specified Impurity B [ep]
17. Loratadine Related Compound C Rs [usp]
18. Ec 700-257-5
19. Schembl3839936
20. Dtxsid40185183
21. Bcp31805
22. Zinc2569976
23. Akos005067771
24. Ac-26332
25. As-12268
26. Loratadine Impurity, 8-chloro-6,11-dihydro-5h-benzo(5,6)cyclohepta(1,2-b)pyridin-11-one- [usp]
27. Sy053120
28. Loratadine Impurity B [ep Impurity]
29. Cs-0022883
30. Ft-0638960
31. Loratadine Ep Impurity B ( Usp Rc C)
32. A18619
33. Loratadine Related Compound C [usp-rs]
34. 251c419
35. Loratadine Related Compound C [usp Impurity]
36. Q27294379
37. 8-chloro-10,11-dihydro-4-aza-5h-dibenzo-(a,d)cycloheptan-5one
38. 8-chloro-10,11-dihydro-4-aza-5h-dibenzo[a,d]cyclohepten-5-one
39. 8-chloro-10,11-dihydro-4 Aza-5h-benzo[a,d] Cycloheptan-5-one
40. 8-chloro-5,6-dihydro-11 H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-one
41. 8-chloro-5,6-dihydro-11h-benzo [5,6]cyclohepta [1,2-b]pyrid-11-one
42. 8-chloro-5,6-dihydro-11h-benzo[5,6]cyclo Hepta[1,2-b]pyridin-11-one
43. 8-chloro-5,6-dihydro-11h-benzo[5,6]cyclohepta[1,2-b]pyrid-11-one
44. Loratadine Impurity, 8-chloro-6,11-dihydro-5h-benzo(5,6)cyclohepta(1,2-b)pyridin-11-one- [usp Impurity]
| Molecular Weight | 243.69 g/mol |
|---|---|
| Molecular Formula | C14H10ClNO |
| XLogP3 | 3.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 243.0450916 g/mol |
| Monoisotopic Mass | 243.0450916 g/mol |
| Topological Polar Surface Area | 30 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 307 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |






