



1. 27710-82-3
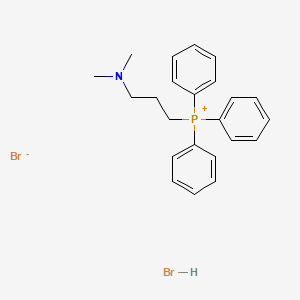
2. (3-(dimethylamino)propyl)triphenylphosphonium Bromide Hydrobromide
3. (3-dimethylaminopropyl)triphenylphosphonium Bromide Hydrobromide
4. Mfcd00077740
5. Ec 608-135-2
6. Schembl246032
7. Dtxsid20635333
8. Akos015889870
9. Ab02982
10. Ac-4737
11. Am84872
12. [3-(dimethylamino)propyl](triphenyl)phosphanium Bromide--hydrogen Bromide (1/1/1)
13. Phosphonium, [3-(dimethylamino)propyl]triphenyl-, Bromide, Hydrobromide (1:1:1)
14. (2-carboxyphenyl)iminodiaceticacid
15. Sy043358
16. Db-016410
17. Cs-0151289
18. Ft-0638717
19. Dimethylaminopropyltriphenylphosphonium Bromide Hbr
20. (3-dimethylaminopropyl)-triphenylphosphoniumbromide Hbr
21. (3-(dimethylamino)propyl)triphenylphosphoniumbromidehydrobromide
22. 3-(dimethylamino)propyltriphenylphosphonium Bromide Hydrobromide
23. (3-(dimethylamino)propyl)triphenyl-phosphonium Bromide Hydrobromide
24. (3-dimethylaminopropyl)-triphenylphosphonium Bromide Hydrobromide
25. (3-(dimethylamino)propyl)triphenylphosphonium Bromide Hydrobromide Salt
| Molecular Weight | 509.3 g/mol |
|---|---|
| Molecular Formula | C23H28Br2NP |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 7 |
| Exact Mass | 509.03056 g/mol |
| Monoisotopic Mass | 507.03261 g/mol |
| Topological Polar Surface Area | 3.2 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 317 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |






