




1. 130049-82-0
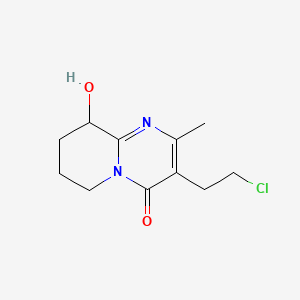
2. 3-(2-chloroethyl)-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4h-pyrido[1,2-a]pyrimidine-4-one
3. 3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4h-pyrido[1,2-a]pyrimidin-4-one
4. 3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one
5. Mfcd09835552
6. Paliperidone Impurity 08
7. 4m62ixl9x4
8. 4h-pyrido[1,2-a]pyrimidin-4-one, 3-(2-chloroethyl)-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-
9. 3-(2-chloroethyl)-2-methyl-9-hydroxy-6,7,8,9-tetrahydro-4h-pyrido (1,2-a) Pyrimidin-4-one
10. 4h-pyrido(1,2-a)pyrimidin-4-one, 3-(2-chloroethyl)-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-
11. Paliperidone Related Compound C
12. 3-(2-chloroethyl)-2-methyl-9-hydroxy-6,7,8,9-tetrahydro-4h-pyrido [1,2-a] Pyrimidin-4-one
13. 3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4h-pyrido(1,2-a)pyrimidin-4-one
14. Paliperidone Impurity N
15. Unii-4m62ixl9x4
16. Schembl530941
17. Dtxsid00573961
18. 3-(2,2,2-trimethylhydrazinyl)propenoic Acid Methyl Ester Bromide
19. Amy40183
20. Bcp11296
21. Cs-b0009
22. Akos015850555
23. Ac-1499
24. Ds-11346
25. Sy019547
26. Db-028213
27. Ft-0649316
28. Paliperidone Related Compound C [usp-rs]
29. 049c820
30. A806009
31. Q-102531
32. Q-200327
33. Paliperidone Related Compound C [usp Impurity]
34. 3-(2-chloroethyl)-2-methyl-9-hydroxy-6,7,8,9-tetrahydro-pyrido[1,2-a]pyrimidin-4-one
35. 3-(2-chloroethyl)-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4h-pyrido[1,2-a]-pyrimidin-4-one
36. 3-(2-chloroethyl)-9-hydroxy-6,7,8,9-tetrahydro-2-methyl-4h-pyrido[1,2-a]pyrimidin-4-one
37. 3-(2-chloroethyl)-9-hydroxy-6,7,8,9-tetrahydro-2-methyl-4h-pyrido[1,2-a]pyrimidine-4-one
38. 3-(2-chloroethyl)-9-hydroxy-6,7,8.9-tetrahydro-2-methyl-4h-pyrido[1,2-a]pyrimidine-4-one
39. 3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4h-pyrido-[1,2-a]-pyrimidin-4-one
40. 3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one;3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4h-pyrido[1,2-a]pyrimidin-4-one
41. 3-(2-chloroethyl)-9-hydroxy-6,7,8,9-tetrahydro-2-methyl-4h-pyrido[1,2-a]-pyrimidine-4-one
42. 9-hydroxy-3-(2-chloroethyl)-2-methyl-6,7,8,9-tetrahydro-4h-pyrido[1,2-alpha]pyrimidin-4-one
| Molecular Weight | 242.70 g/mol |
|---|---|
| Molecular Formula | C11H15ClN2O2 |
| XLogP3 | 0.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 242.0822054 g/mol |
| Monoisotopic Mass | 242.0822054 g/mol |
| Topological Polar Surface Area | 52.9 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 376 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |







