




1. 122188-02-7
2. Etodolac, Methylated
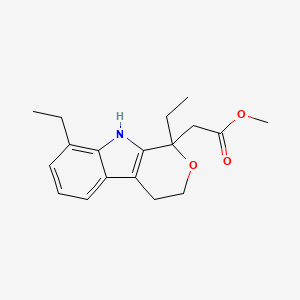
3. 1,8-diethyl-1,3,4,9-tetrahydro-pyrano[3,4-b]indole-1-acetic Acid Methyl Ester
4. Methyl 2-(1,8-diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl)acetate
5. Methyl 2-(1,8-diethyl-4,9-dihydro-3h-pyrano[3,4-b]indol-1-yl)acetate
6. Pyrano[3,4-b]indole-1-acetic Acid, 1,8-diethyl-1,3,4,9-tetrahydro-, Methyl Ester
7. 9zje4h85sv
8. Etodolac Impurity K [ep Impurity]
9. Methyl 2-(1,8-diethyl-1,3,4,9-tetrahydropyrano(3,4-b)indol-1-yl)acetate
10. Methyl ((1rs)-1,8-diethyl-1,3,4,9-tetrahydropyrano(3,4-b)indol-1-yl)acetate
11. Pyrano(3,4-b)indole-1-acetic Acid, 1,8-diethyl-1,3,4,9-tetrahydro-, Methyl Ester
12. Methyl 2-[(1rs)-1,8-diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl]acetate (etodolac Methyl Ester)
13. Etodolac Impurity K
14. Etodolacmethylester
15. Rac-etodolac Methyl Ester
16. Unii-9zje4h85sv
17. Schembl6629123
18. Dtxsid60881392
19. Mfcd02684434
20. Akos015895577
21. Methyl2-(1,8-diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl)acetate
22. Ac-4237
23. Ac-7752
24. Bs-42122
25. Db-015609
26. Ft-0642978
27. Ft-0655516
28. 880e315
29. A804854
30. Q-201100
31. Methyl1,8-diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indole-1-acetate
32. Methyl 1,8-diethyl-1,3,4,9-tetrahydropyrano (3,4-b)-indole-1-acetate
33. Methyl 1,8-diethyl-1,3,4,9-tetrahydropyrano(3,4-b)-indole-1-acetate
34. Methyl 2-(1,8-diethyl-4,9-dihydro-3h-pyrano[3,4-b]indol-1-yl)ethanoate
35. 1,8-diethyl-1,3,4,9-tetrahydro-pyrano [3,4-b] Indole-1-acetic Acid Methyl Ester
36. 1,8-diethyl-1,3,4,9-tetrahydropyrano-3,4-$b-indole-1-acetic Acid Methyl Ester
37. 2-(1,8-diethyl-4,9-dihydro-3h-pyrano[3,4-b]indol-1-yl)acetic Acid Methyl Ester
| Molecular Weight | 301.4 g/mol |
|---|---|
| Molecular Formula | C18H23NO3 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 5 |
| Exact Mass | 301.16779360 g/mol |
| Monoisotopic Mass | 301.16779360 g/mol |
| Topological Polar Surface Area | 51.3 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 413 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |






