


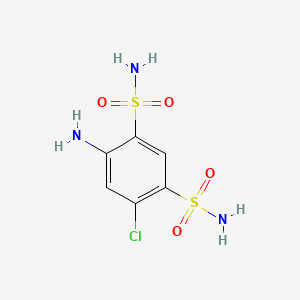

1. 2-acbds
2. 2-amino-4-chloro-m-benzenedisulfonamide
1. 121-30-2
2. 4-amino-6-chlorobenzene-1,3-disulfonamide
3. Chloraminophenamide
4. Idorese
5. Salmid
6. Salamid
7. Chloroaminophenamide
8. 1,3-benzenedisulfonamide, 4-amino-6-chloro-
9. 3-chloro-4,6-disulfamoylaniline
10. 5-chloro-2,4-disulfamoylaniline
11. 5-chloro-2,4-disulfamylaniline
12. 4-amino-6-chloro-m-benzenedisulfonamide
13. Su 5683
14. 4-amino-6-chlorobenzene-1,3-disulphonamide
15. M-benzenedisulfonamide, 4-amino-6-chloro-
16. 3-chloroaniline-4,6-disulfonamide
17. Mfcd00007933
18. 3a52o8yrej
19. Chebi:3602
20. Chembl266240
21. 4-amino-6-chloro-benzene-1,3-disulfonamide
22. Nsc-93772
23. 2-amino-4-chloro-m-benzenedisulfonamide
24. 1,3-disulfonamide, 4-amino-6-chlorobenzene-
25. 4-amino-6-chlorobenzene-1,3-disulphonamide (salamide)
26. Benzothiadiazine Related Compound A
27. 4-amino-6-chloro-1,3-benzendisulfonamide
28. 1184995-49-0
29. Unii-3a52o8yrej
30. 2pov
31. I7b
32. Einecs 204-463-1
33. Nsc 93772
34. 4-amino-6-chloro-benzene-1,3-disulfonic Acid Diamide
35. 1, 4-amino-6-chloro-
36. Ec 204-463-1
37. Mls006011405
38. Schembl310205
39. Chloraminophenamide [mi]
40. Dtxsid1059521
41. Bdbm10867
42. Ihjcxvzdysxxft-uhfffaoysa-
43. 5-chloro-2,4-disulfamyl Aniline
44. M-chloroaniline-4,6-disulfonamide
45. Albb-025760
46. Bcp24485
47. Nsc93772
48. Zinc1530783
49. Ac-036
50. Ac1883
51. Bbl003489
52. Stk803151
53. 5-chloro-2,4-bis(sulfonamide)aniline
54. Akos000120232
55. Aromatic/heteroaromatic Sulfonamide 12
56. Sb82332
57. Su-5683
58. Altizide Impurity A [ep Impurity]
59. As-12956
60. Smr002530008
61. Sy051012
62. 1-amino-3-chloro-4,6-benzenedisulfonamide
63. 4-amino-6-chlorobenzene-1-3-disulfonamide
64. Cs-0075543
65. Ft-0617536
66. Ft-0661667
67. 5(3)-chloroaniline-2,4(4,6)-disulfonamide
68. 2-amino-4-chloro-5-sulfamoylbenzenesulfonamide
69. C07460
70. 1-amino-5-chloro-2,4-benzenedisulfonamide
71. 4-amino-6-chloro-1,3-benzenedisulfonamide, 98%
72. 4-amino-6-chloro-1,3-benzenedisulfonamide-[d6]
73. A804710
74. Sr-01000944745
75. Benzothiadiazine Related Compound A [usp-rs]
76. Hydrochlorothiazide Impurity B [ep Impurity]
77. Sr-01000944745-1
78. W-108458
79. 4-amino-6-chlorobenzen-1,3-disulphonamide(salamide)
80. Q27106146
81. F0001-0341
82. Benzothiadiazine Related Compound A [usp Impurity]
83. 3-chloroaniline-4,6-disulfonamide, 4-amino-6-chlorobenzene-1,3-disulfonamide
84. Benzothiadiazine Related Compound A, United States Pharmacopeia (usp) Reference Standard
85. 4-amino-6-chloro-1,3-benzenedisulfonamide; (benzothiadiazine Related Compound A), Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 285.7 g/mol |
|---|---|
| Molecular Formula | C6H8ClN3O4S2 |
| XLogP3 | -0.5 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 2 |
| Exact Mass | 284.9644758 g/mol |
| Monoisotopic Mass | 284.9644758 g/mol |
| Topological Polar Surface Area | 163 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 451 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |




