




1. 119018-29-0
2. Glimepiride Sulfonamide
3. Glimepiride Related Compound B
4. Glimepiride Sulphonamide
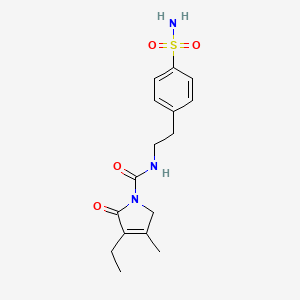
5. 4-ethyl-3-methyl-5-oxo-n-[2-(4-sulfamoylphenyl)ethyl]-2h-pyrrole-1-carboxamide
6. 612913u5l6
7. 3-ethyl-4-methyl-2-oxo-n-[2-(4-sulfamoylphenyl)ethyl]-2,5-dihydro-1h-pyrrole-1-carboxamide
8. 4-(2-((3-ethyl-4-methyl-2-oxo-3-pyrrolin-1-yl)carboxamido)ethyl)benzenesulfonamide
9. 3-ethyl-4-methyl-2-oxo-n-[2-(4-sulphamoylphenyl)ethyl]-2,3-dihydro-1h-pyrrole-1-carboxamide (glimepiride Sulphonamide)
10. Unii-612913u5l6
11. Ec 411-850-0
12. Oprea1_386350
13. Schembl14371709
14. Dtxsid20152297
15. Amy33442
16. Bcp10472
17. Usp Glimepiride Related Compound B
18. Mfcd02955393
19. Stk993693
20. Zinc21298457
21. Akos005657772
22. Ac-1493
23. Cs-w022064
24. Gs-3185
25. 3-ethyl-4-methyl-2-oxo-n-(4-sulfamoylphenethyl)-2,5-dihydro-1h-pyrrole-1-carboxamide
26. 4-(2-((3-ethyl-4-methyl-2-oxo-pyrrolin-1-yl)carboxamido)ethyl)benzenesulfonamide)
27. 3-ethyl-4-methyl-2-oxo-2,5-dihydro-pyrrole-1-carboxylic Acid [2-(4-sulfamoyl-phenyl)-ethyl]-amide
28. N-[2-[4-(aminosulfonyl)phenyl]ethyl]-3-ethyl-2,5-dihydro-4-methyl-2-oxo-1h-pyrrole-1-carboxamide
29. Ft-0642911
30. Glimepiride Impurity B [ep Impurity]
31. C71616
32. Glimepiride Related Compound B [usp-rs]
33. 018e290
34. A804150
35. J-004054
36. Glimepiride Related Compound B [usp Impurity]
37. Q27263274
38. 4-[2-(2-oxo-3-ethyl-4-methyl-3-pyrroline-1-ylcarbonylamino)ethyl]benzenesulfonamide
39. 4-[2-(3-ethyl-4-methyl-2-oxo-3-pyrrolidine-1- Carboxamido)ethyl]benzenesulfonamide
40. 4-[2-[(3-ethyl-4-methyl-2-oxo-3-pyrrolin-1-yl)carboxamido]ethyl] Benzenesulfonamide
41. 1h-pyrrole-1-carboxamide, N-(2-(4-(aminosulfonyl)phenyl)ethyl)-3-ethyl-2,5-dihydro-4-methyl-2-oxo
42. 1h-pyrrole-1-carboxamide, N-[2-[4-(aminosulfonyl)phenyl]ethyl]-3-ethyl-2,5-dihydro-4-methyl-2-oxo-
| Molecular Weight | 351.4 g/mol |
|---|---|
| Molecular Formula | C16H21N3O4S |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 5 |
| Exact Mass | 351.12527733 g/mol |
| Monoisotopic Mass | 351.12527733 g/mol |
| Topological Polar Surface Area | 118 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 628 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |



