



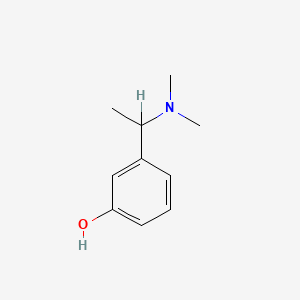

1. 3-(1-dimethylaminoethyl)phenol
2. Nap 226-90
3. Nap-226-90
4. Nap226-90
1. 105601-04-5
2. 3-(1-(dimethylamino)ethyl)phenol
3. 3-(1-(dimethylamino)ethyl]phenol
4. Phenol, 3-[1-(dimethylamino)ethyl]-
5. 3-[(1s)-1-(dimethylaminoethyl)]phenol
6. 3-(1-dimethylaminoethyl)phenol
7. Mfcd08272688
8. Phenol,3-[1-(dimethylamino)ethyl]-
9. Mfcd06656491
10. Dipropylmaleate
11. Rivastigmine Intermediates
12. Schembl43343
13. Bdbm10630
14. Dtxsid20328334
15. 3-(1-dimethylamino-ethyl)-phenol
16. Hms3604c09
17. Stl450948
18. Akos015850703
19. Ac-3488
20. Mb03878
21. Sb44524
22. Sb76291
23. Sy067144
24. Sy102287
25. (+/-)-3-(1-(dimethylamino)ethyl)phenol
26. Db-012764
27. Am20080966
28. Cs-0154112
29. D5326
30. Ft-0642603
31. Ft-0649363
32. Ft-0658969
33. Ft-0667382
34. Ft-0672608
35. 3-[1-(dimethylamino)ethyl]phenol, Aldrichcpr
36. 601d045
37. A801275
38. Q-101325
39. N,n-dimethyl-n-[1-(3'-hydroxyphenyl)ethyl]amine;zns 114-666
| Molecular Weight | 165.23 g/mol |
|---|---|
| Molecular Formula | C10H15NO |
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 165.115364102 g/mol |
| Monoisotopic Mass | 165.115364102 g/mol |
| Topological Polar Surface Area | 23.5 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 136 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |





