Overview of Small molecule API development & more on CDMOs & CMOs offering small molecule drug substances synthesis & manufacturing services.Q1. What are small molecule APIs, and how do they differ from large molecule APIs?Small molecules are simple chemical compounds that are obtained through chemical synthesis with molecular weights below 900 Daltons or ranging from 0.1 to 1.0 kilodalton (kDa). Small molecules due to their small size, diffuse through the cell membrane and reach the intracellular targets or desired site of action.Small molecule drugs frequently possess a well-defined mechanism of action and are designed to specifically interact with a particular enzyme or receptor, so as to modify a biological pathway or function.These small molecule APIs are administered into the body through oral route or via injections. Since oral delivery offers greater patient compliance and lower cost of administration in comparison to injectables, small molecule APIs are usually formulated into oral dosage forms.Small molecules account for 90% of the pharmaceutical market as they are patient-friendly and easy to administer via oral route. These compounds are notably smaller than large molecules (biological molecules) such as proteins. Small molecule APIs have lower molecular weight while large molecule APIs range from ~3,000 to 150,000 Da.In comparison to small molecule APIs, large molecules are difficult to characterize and have a more complicated structure. Small molecules are quickly absorbed into the bloodstream and subsequently enter cells, where they interact with other molecules such as proteins. In contrast, large molecules provide site-specific cellular binding without affecting healthy cells.Small molecule APIs offer enhanced stability and can be readily formulated into tablets, capsules, inhalations, injectables, and suppositories. Whereas large molecule APIs (biological APIs) exhibit relatively lower stability and degrade in the gastrointestinal tract when taken orally. Therefore, large molecules (biologics) are typically administered via injections or infusions.Small molecules generally comprise lipids, amino acids, fatty acids, phenolic compounds, and alkaloids. While large molecules also known as biologics or biologicals, or biopharmaceuticals comprise proteins, sugars, nucleic acids, cells, tissues, blood and its derivatives, vaccines, gene therapies, immunotherapies, ADCs, etc.Q2. How are emerging technologies and innovations influencing the research, development, and manufacturing of small molecule APIs in the pharmaceutical industry?Small molecules are the mainstay of the pharmaceutical industry as they are used in the treatment of certain complex and life-threatening conditions. These are becoming the preferred choice of researchers and science professionals due to their low molecular weight and simple chemical structure. Moreover, small molecules have predictable pharmacokinetic and pharmacodynamic attributes, and drug-drug interactions and are more stable and easy to use which makes their dosage regime simpler and straightforward.The small molecule API market is governed by several factors including increased research and developmental activities, rising predominance of chronic diseases, and increased requirements of generics. Advanced developmental technologies coupled with cost-effective treatments, also contribute to the small molecule API development.Emerging trends and technologies might include quantum computing, artificial intelligence, precision medicine, materials sciences and particle engineering, advanced analytical tools, etc. Additionally, continuous manufacturing also contributes to the small molecule API development and manufacturing.Therefore these technologies and innovations inclusively enhance the efficiency, precision, and sustainability of small molecule active pharmaceutical ingredients (APIs) research, development, and manufacturing.Q3. What are the key benefits of outsourcing small molecule API development to CDMOs?The research, development, and manufacturing of small molecule APIs can be complex sometimes and require special expertise in terms of advanced equipment and experienced personnel. Pharmaceutical companies therefore find the necessity to outsource their small molecule APIs to contract research organizations (CROs), contract development and manufacturing organizations (CDMOs), and contract manufacturing organizations (CMOs).CDMOs support pharmaceutical companies at all stages of research and development. The small molecule CDMOs offer competencies in API development, manufacturing, and regulatory compliance. Outsourcing R&D and manufacturing to CDMOs offers numerous advantages for pharmaceutical companies, including increased flexibility, quicker market entry, risk mitigation, and cost-effectiveness, allowing them to reduce financial exposure to small molecule APIs.Outsourcing to CDMOs remains a vital strategy for easing development challenges. Therefore, outsourcing small molecule API development to contract development and manufacturing organizations (CDMOs) offers several advantages that include:Advanced Expertise and Accelerated Developmental Timelines: CDMOs usually have specialized skills for small molecule API development and manufacturing and employ dedicated teams to enhance and streamline the synthesis process. They also have expertise, advanced technologies, and well-established procedures, for accelerating the small molecule API development timelines.Lower Operational Cost: CDMOs can lower operational costs by leveraging advanced infrastructure and simplifying processes, ultimately reducing costs for small molecule API development and manufacturing for the client.Advanced Technologies: CDMOs have state-of-the-art equipment and methodologies, offering access to cutting-edge technologies such as biocatalysis and flow chemistry that could be expensive for individual companies for the development of small molecule APIs.Flexibility and Scale-Up: CDMOs provide adaptable production capacities, allowing easy scalability to adjust to different small molecule production volumes, catering to the evolving requirements of their clients.Risk Mitigation: CDMOs often have specialized entities and technologies, enabling them to concentrate on core strengths and alleviating the complexities of small molecule API development processes.Enhanced Inventory Management: CDMOs can manage the inventory for small molecule raw materials, intermediates, and final products, guaranteeing adequate stock to meet demand.Reduced Time-to-Market: CDMOs’ expertise and resources frequently lead to quicker development timelines, accelerating the introduction of new small molecule APIs to market.Regulatory Compliance: CDMOs are well-versed in small molecule API regulatory requirements, aiding in compliance with industry standards.The CDMO for small molecule APIs offers advantages in enhancing efficiency, accelerating timelines, and reducing research and development expenses. Several pharmaceutical contract development and manufacturing organizations (CDMOs) and contract manufacturing organizations (CMOs) offer synthesis of small molecule APIs, drug substance development services, and small molecule API contract manufacturing from cGMP clinical to commercial manufacturing. Therefore, with the rising difficulties and quality standards, companies are considering outsourcing APIs to small molecule CDMOs.Q4. What are the leading CDMOs offering small molecule API development services?Various organizations, such as pharmaceutical contract development and manufacturing organizations (CDMOs) and contract manufacturing organizations (CMOs), can offer small molecule API development and manufacturing services. These are also known as small molecule API CDMOs or CMOs that might also offer material for clinical trial services or clinical supplies and can develop and scale up small molecules.Small molecule contract development and manufacturing organizations offer drug substances that are in greater demand due to their success in treating a variety of illnesses and disorders. Small molecule API development and manufacturing is still a challenging, multi-step process requiring a variety of raw materials with various chemical transformations, and operations. Specialized expertise of small molecule API CMOs and CDMOs is required for API development and manufacturing for research and cGMP clinical and commercial supply.Recently, CDMOs for small molecule APIs have gained importance because of increased R&D costs, requirements for special competencies, and small molecule API development and manufacturing services they offer. Due to this reason, there has been a surge in outsourcing APIs to small molecule CDMOs.The market for small molecule API contract manufacturing and development is constantly expanding. Several small molecule CDMOs are providing comparable pharmaceutical custom development and manufacturing services. These small molecule API CMOs and CDMOs have years of experience in pharmaceutical development and commercial manufacturing of small molecule drugs along with material supply for clinical trial services. Some of the top small molecule contract development and manufacturing organizations (CDMOs) with pharmaceutical custom development capabilities are explained below:Axplora: Axplora is a CDMO for small molecule APIs that offers a full range of pharmaceutical custom development services for small molecule active pharmaceutical ingredients and biomolecules. It is a small molecule API CDMO providing cGMP production and drug substance development services for commercial supply.Aurigene Pharmaceutical Services: Aurigene Pharmaceutical Services is a pharmaceutical contract development and manufacturing organization that provides drug substance development services. It is a custom pharma CDMO and small molecule API CMO that offers pharmaceutical development and cGMP clinical and commercial services for drug substances, including small molecules, HPAPIs, peptides, carbohydrates, and steroids.Pfizer CentreOne: Pfizer CentreOne is a CMO and a CDMO for small molecule APIs specializing in small molecule API development and manufacturing. Its custom pharma CDMO and CMO services include the synthesis of small molecule APIs, small molecule API contract manufacturing, and cGMP clinical and commercial supply.Polpharma: Polpharma is a pharmaceutical contract development and manufacturing organization supporting small molecule API development. It is a CDMO for small molecule APIs offering small molecule API contract manufacturing and cGMP clinical and commercial services.EUROAPI: EUROAPI is a global small molecule API CDMO that offers drug substance development services and synthesis of small molecule active pharmaceutical ingredients. Its custom pharma CDMO and CMO services include small molecule API development along with commercial manufacturing of API via organic synthesis and fermentation process.Aspen API: Aspen API is a CDMO for small molecule APIs that has expertise in pharmaceutical development and manufacturing of APIs through chemical synthesis and microbial fermentation. It is a contract manufacturer that offers CMO services for scale-up and commercial manufacturing along with the synthesis of small molecule APIs, high-potency APIs (HPAPIs), peptides, steroids, etc.
Impressions: 53



![]() Comment
Comment




![]() Comment
Comment





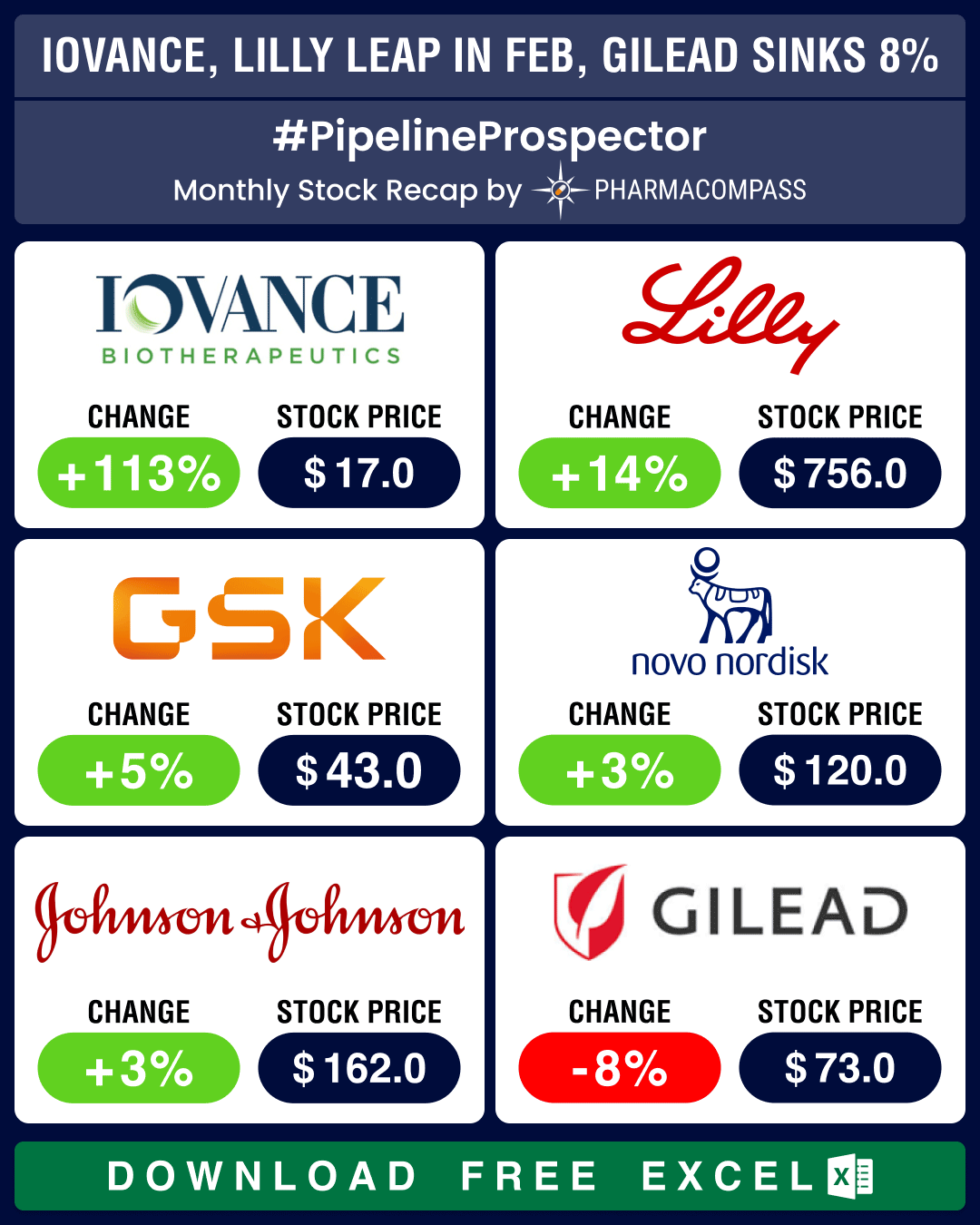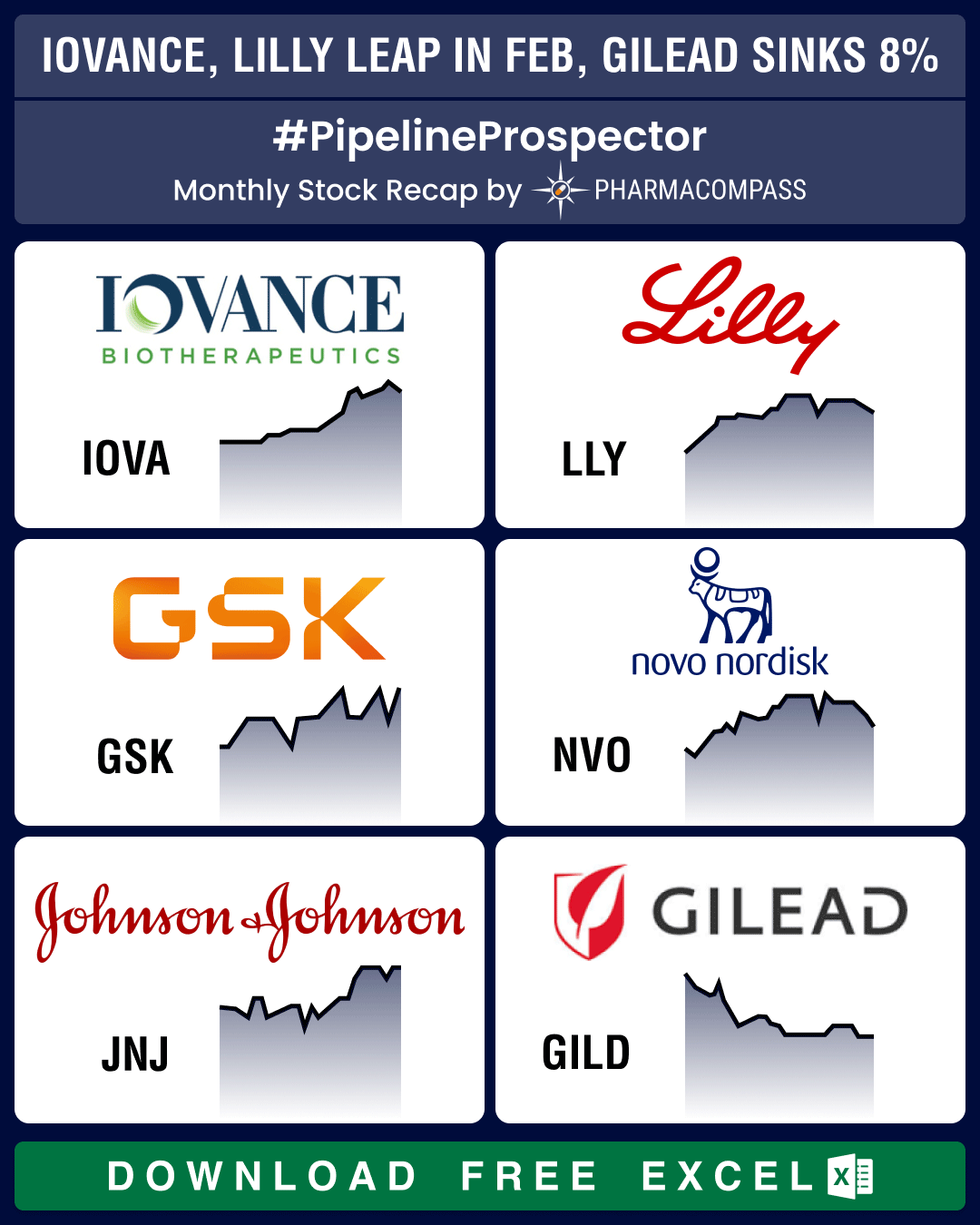


 Market Place
Market Place Post Enquiry
Post Enquiry